Interaction Between Pannexin 1 and Caveolin-1 in Smooth Muscle Can Regulate Blood Pressure
- PMID: 30026274
- PMCID: PMC6202122
- DOI: 10.1161/ATVBAHA.118.311290
Interaction Between Pannexin 1 and Caveolin-1 in Smooth Muscle Can Regulate Blood Pressure
Abstract
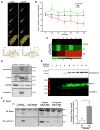
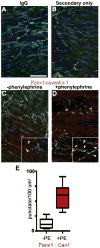
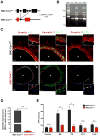
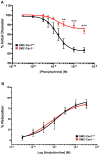
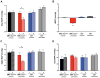
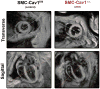
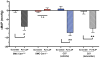
Objective- Sympathetic nerve innervation of vascular smooth muscle cells (VSMCs) is a major regulator of arteriolar vasoconstriction, vascular resistance, and blood pressure. Importantly, α-adrenergic receptor stimulation, which uniquely couples with Panx1 (pannexin 1) channel-mediated ATP release in resistance arteries, also requires localization to membrane caveolae. Here, we test whether localization of Panx1 to Cav1 (caveolin-1) promotes channel function (stimulus-dependent ATP release and adrenergic vasoconstriction) and is important for blood pressure homeostasis. Approach and Results- We use in vitro VSMC culture models, ex vivo resistance arteries, and a novel inducible VSMC-specific Cav1 knockout mouse to probe interactions between Panx1 and Cav1. We report that Panx1 and Cav1 colocalized on the VSMC plasma membrane of resistance arteries near sympathetic nerves in an adrenergic stimulus-dependent manner. Genetic deletion of Cav1 significantly blunts adrenergic-stimulated ATP release and vasoconstriction, with no direct influence on endothelium-dependent vasodilation or cardiac function. A significant reduction in mean arterial pressure (total=4 mm Hg; night=7 mm Hg) occurred in mice deficient for VSMC Cav1. These animals were resistant to further blood pressure lowering using a Panx1 peptide inhibitor Px1IL2P, which targets an intracellular loop region necessary for channel function. Conclusions- Translocalization of Panx1 to Cav1-enriched caveolae in VSMCs augments the release of purinergic stimuli necessary for proper adrenergic-mediated vasoconstriction and blood pressure homeostasis.
Keywords: Pannexin 1; adrenergic agents; blood pressure; caveolae; muscle, smooth.
Conflict of interest statement
Figures
References
-
- Christensen KL, Mulvany MJ. Location of resistance arteries. Journal of vascular research. 2001;38(1):1–12. - PubMed
-
- Bennett MR. Autonomic neuromuscular transmission at a varicosity. Progress in neurobiology. 1996;50(5–6):505–532. - PubMed
-
- Burnstock G, Ralevic V. Purinergic Signaling and Blood Vessels in Health and Disease. Pharmacological Reviews. 2013;66(1):102–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

