Arsenic trioxide is required in the treatment of newly diagnosed acute promyelocytic leukemia. Analysis of a randomized trial (APL 2006) by the French Belgian Swiss APL group
- PMID: 30026341
- PMCID: PMC6269295
- DOI: 10.3324/haematol.2018.198614
Arsenic trioxide is required in the treatment of newly diagnosed acute promyelocytic leukemia. Analysis of a randomized trial (APL 2006) by the French Belgian Swiss APL group
Abstract
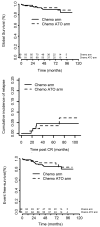
In standard-risk acute promyelocytic leukemia, recent results have shown that all-trans retinoic acid plus arsenic trioxide combinations are at least as effective as classical all-trans retinoic acid plus anthracycline-based chemotherapy while being less myelosuppressive. However, the role of frontline arsenic trioxide is less clear in higher-risk acute promyelocytic leukemia, and access to arsenic remains limited for front-line treatment of standard-risk acute promyelocytic leukemia in many countries. In this randomized trial, we compared arsenic, all-trans retinoic acid and the "classical" cytarabine for consolidation treatment (after all-trans retinoic acid and chemotherapy induction treatment) in standard-risk acute promyelocytic leukemia, and evaluated the addition of arsenic during consolidation in higher-risk disease. Patients with newly diagnosed acute promyelocytic leukemia with a white blood cell count <10x109/L, after an induction treatment consisting of all-trans retinoic acid plus idarubicin and cytarabine, received consolidation chemotherapy with idarubicin and cytarabine, arsenic or all-trans retinoic acid. Patients with a white blood cell count >10x109/L received consolidation chemotherapy with or without arsenic. Overall, 795 patients with acute promyelocytic leukemia were enrolled in this trial. Among those with standard-risk acute promyelocytic leukemia (n=581), the 5-year event-free survival rates from randomization were 88.7%, 95.7% and 85.4% in the cytarabine, arsenic and all-trans retinoic acid consolidation groups, respectively (P=0.0067), and the 5-year cumulative incidences of relapse were was 5.5%, 0% and 8.2%. (P=0.001). Among those with higher-risk acute promyelocytic leukemia (n=214), the 5-year event-free survival rates were 85.5% and 92.1% (P=0.38) in the chemotherapy and chemotherapy plus arsenic groups, respectively, and the corresponding 5-year cumulative incidences of relapse were 4.6% and 3.5% (P=0.99). Given the prolonged myelosuppression that occurred in the chemotherapy plus arsenic arm, a protocol amendment excluded cytarabine during consolidation cycles in the chemotherapy plus arsenic group, resulting in no increase in relapse. Our results therefore advocate systematic introduction of arsenic in the first-line treatment of acute promyelocytic leukemia, but probably not concomitantly with intensive chemotherapy, a situation in which we found myelosuppression to be significant. (ClinicalTrials.gov Identifier: NCT00378365).
Copyright© 2018 Ferrata Storti Foundation.
Figures

References
-
- Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113(9):1875–1891. - PubMed
-
- Lo-Coco F, Avvisati G, Vignetti M, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA group. Blood. 2010;116(17):3171–3179. - PubMed
-
- Adès L, Chevret S, Raffoux E, et al. Long-term follow-up of European APL 2000 trial, evaluating the role of cytarabine combined with ATRA and daunorubicin in the treatment of nonelderly APL patients. Am J Hematol. 2013;88(7):556–559. - PubMed
-
- Sanz MA, Montesinos P, Rayón C, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood. 2010;115(25): 5137–5146. - PubMed
-
- Fenaux P, Chastang C, Chevret S, et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL group. Blood. 1999;94(4): 1192–1200. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

