Chronic Electrical Stimulation Promotes the Excitability and Plasticity of ESC-derived Neurons following Glutamate-induced Inhibition In vitro
- PMID: 30026496
- PMCID: PMC6053382
- DOI: 10.1038/s41598-018-29069-3
Chronic Electrical Stimulation Promotes the Excitability and Plasticity of ESC-derived Neurons following Glutamate-induced Inhibition In vitro
Abstract
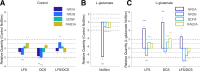
Functional electrical stimulation (FES) is rapidly gaining traction as a therapeutic tool for mediating the repair and recovery of the injured central nervous system (CNS). However, the underlying mechanisms and impact of these stimulation paradigms at a molecular, cellular and network level remain largely unknown. In this study, we used embryonic stem cell (ESC)-derived neuron and glial co-cultures to investigate network maturation following acute administration of L-glutamate, which is a known mediator of excitotoxicity following CNS injury. We then modulated network maturation using chronic low frequency stimulation (LFS) and direct current stimulation (DCS) protocols. We demonstrated that L-glutamate impaired the rate of maturation of ESC-derived neurons and glia immediately and over a week following acute treatment. The administration of chronic LFS and DCS protocols individually following L-glutamate infusion significantly promoted the excitability of neurons as well as network synchrony, while the combination of LFS/DCS did not. qRT-PCR analysis revealed that LFS and DCS alone significantly up-regulated the expression of excitability and plasticity-related transcripts encoding N-methyl-D-aspartate (NMDA) receptor subunit (NR2A), brain-derived neurotrophic factor (BDNF) and Ras-related protein (RAB3A). In contrast, the simultaneous administration of LFS/DCS down-regulated BDNF and RAB3A expression. Our results demonstrate that LFS and DCS stimulation can modulate network maturation excitability and synchrony following the acute administration of an inhibitory dose of L-glutamate, and upregulate NR2A, BDNF and RAB3A gene expression. Our study also provides a novel framework for investigating the effects of electrical stimulation on neuronal responses and network formation and repair after traumatic brain injury.
Conflict of interest statement
Dr. Stice has a financial interest in ArunA Biomedical Inc., a company that develops cell-free biologics for CNS therapies. All other authors declare no potential conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

