Reversing wrinkled skin and hair loss in mice by restoring mitochondrial function
- PMID: 30026579
- PMCID: PMC6053453
- DOI: 10.1038/s41419-018-0765-9
Reversing wrinkled skin and hair loss in mice by restoring mitochondrial function
Abstract
Mitochondrial DNA (mtDNA) depletion is involved in mtDNA depletion syndromes, mitochondrial diseases, aging and aging-associated chronic diseases, and other human pathologies. To evaluate the consequences of depletion of mtDNA in the whole animal, we created an inducible mtDNA-depleter mouse expressing, in the polymerase domain of POLG1, a dominant-negative mutation to induce depletion of mtDNA in various tissues. These mice showed reduced mtDNA content, reduced mitochondrial gene expression, and instability of supercomplexes involved in oxidative phosphorylation (OXPHOS) resulting in reduced OXPHOS enzymatic activities. We demonstrate that ubiquitous depletion of mtDNA in mice leads to predominant and profound effects on the skin resulting in wrinkles and visual hair loss with an increased number of dysfunctional hair follicles and inflammatory responses. Development of skin wrinkle was associated with the significant epidermal hyperplasia, hyperkeratosis, increased expression of matrix metalloproteinases, and decreased expression of matrix metalloproteinase inhibitor TIMP1. We also discovered markedly increased skin inflammation that appears to be a contributing factor in skin pathology. Histopathologic analyses revealed dysfunctional hair follicles. mtDNA-depleter mice also show changes in expression of aging-associated markers including IGF1R, KLOTHO, VEGF, and MRPS5. mtDNA-repleter mice showed that, by turning off the mutant POLG1 transgene expression, mitochondrial function, as well as the skin and hair pathology, is reversed to wild-type level. To our knowledge that restoration of mitochondrial functions can reverse the skin and hair pathology is unprecedented.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


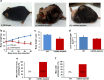





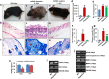
Comment in
-
Mitochondrial DNA keeps you young.Cell Death Dis. 2018 Sep 24;9(10):992. doi: 10.1038/s41419-018-1045-4. Cell Death Dis. 2018. PMID: 30250200 Free PMC article. No abstract available.
Similar articles
-
Exercise-induced mitochondrial p53 repairs mtDNA mutations in mutator mice.Skelet Muscle. 2016 Jan 31;6:7. doi: 10.1186/s13395-016-0075-9. eCollection 2016. Skelet Muscle. 2016. Retraction in: Skelet Muscle. 2021 Mar 30;11(1):8. doi: 10.1186/s13395-021-00264-7. PMID: 26834962 Free PMC article. Retracted.
-
Small molecules restore mutant mitochondrial DNA polymerase activity.Nature. 2025 Jun;642(8067):501-507. doi: 10.1038/s41586-025-08856-9. Epub 2025 Apr 9. Nature. 2025. PMID: 40205042 Free PMC article.
-
Mitochondrial DNA mutations in mutator mice confer respiration defects and B-cell lymphoma development.PLoS One. 2013;8(2):e55789. doi: 10.1371/journal.pone.0055789. Epub 2013 Feb 13. PLoS One. 2013. PMID: 23418460 Free PMC article.
-
Respiratory function decline and DNA mutation in mitochondria, oxidative stress and altered gene expression during aging.Chang Gung Med J. 2009 Mar-Apr;32(2):113-32. Chang Gung Med J. 2009. PMID: 19403001 Review.
-
Mitochondrial theory of aging matures--roles of mtDNA mutation and oxidative stress in human aging.Zhonghua Yi Xue Za Zhi (Taipei). 2001 May;64(5):259-70. Zhonghua Yi Xue Za Zhi (Taipei). 2001. PMID: 11499335 Review.
Cited by
-
Oxidative Phosphorylation Dysfunction Modifies the Cell Secretome.Int J Mol Sci. 2020 May 10;21(9):3374. doi: 10.3390/ijms21093374. Int J Mol Sci. 2020. PMID: 32397676 Free PMC article. Review.
-
NIX initiates mitochondrial fragmentation via DRP1 to drive epidermal differentiation.Cell Rep. 2021 Feb 2;34(5):108689. doi: 10.1016/j.celrep.2021.108689. Cell Rep. 2021. PMID: 33535046 Free PMC article.
-
Mitochondria in cutaneous health, disease, ageing and rejuvenation-the 3PM-guided mitochondria-centric dermatology.EPMA J. 2025 Feb 14;16(1):1-15. doi: 10.1007/s13167-025-00400-z. eCollection 2025 Mar. EPMA J. 2025. PMID: 39991093 Free PMC article.
-
Unraveling mitochondrial influence on mammalian pluripotency via enforced mitophagy.Cell. 2025 Aug 21;188(17):4773-4789.e22. doi: 10.1016/j.cell.2025.05.020. Epub 2025 Jun 10. Cell. 2025. PMID: 40499542
-
Mitochondrial DNA keeps you young.Cell Death Dis. 2018 Sep 24;9(10):992. doi: 10.1038/s41419-018-1045-4. Cell Death Dis. 2018. PMID: 30250200 Free PMC article. No abstract available.
References
-
- Tuppen HA, Blakely EL, Turnbull DM, Taylor RW. Mitochondrial DNA mutations and human disease. Biochim. Biophys. Acta. 1797;113-128:2010. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

