Neuropsychopharmacology of JNJ-55308942: evaluation of a clinical candidate targeting P2X7 ion channels in animal models of neuroinflammation and anhedonia
- PMID: 30026598
- PMCID: PMC6224414
- DOI: 10.1038/s41386-018-0141-6
Neuropsychopharmacology of JNJ-55308942: evaluation of a clinical candidate targeting P2X7 ion channels in animal models of neuroinflammation and anhedonia
Abstract
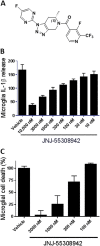
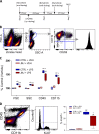
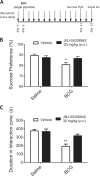
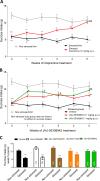
Emerging data continues to point towards a relationship between neuroinflammation and neuropsychiatric disorders. ATP-induced activation of P2X7 results in IL-1β release causing neuroinflammation and microglial activation. This study describes the in-vitro and in-vivo neuropharmacology of a novel brain-penetrant P2X7 antagonist, JNJ-55308942, currently in clinical development. JNJ-55308942 is a high-affinity, selective, brain-penetrant (brain/plasma of 1) P2X7 functional antagonist. In human blood and in mouse blood and microglia, JNJ-55308942 attenuated IL-1β release in a potent and concentration-dependent manner. After oral dosing, the compound exhibited both dose and concentration-dependent occupancy of rat brain P2X7 with an ED50 of 0.07 mg/kg. The P2X7 antagonist (3 mg/kg, oral) blocked Bz-ATP-induced brain IL-1β release in conscious rats, demonstrating functional effects of target engagement in the brain. JNJ-55308942 (30 mg/kg, oral) attenuated LPS-induced microglial activation in mice, assessed at day 2 after a single systemic LPS injection (0.8 mg/kg, i.p.), suggesting a role for P2X7 in microglial activation. In a model of BCG-induced depression, JNJ-55308942 dosed orally (30 mg/kg), reversed the BCG-induced deficits of sucrose preference and social interaction, indicating for the first time a role of P2X7 in the BCG model of depression, probably due to the neuroinflammatory component induced by BCG inoculation. Finally, in a rat model of chronic stress induced sucrose intake deficit, JNJ-55308942 reversed the deficit with concurrent high P2X7 brain occupancy as measured by autoradiography. This body of data demonstrates that JNJ-55308942 is a potent P2X7 antagonist, engages the target in brain, modulates IL-1β release and microglial activation leading to efficacy in two models of anhedonia in rodents.
Conflict of interest statement
Except Drs. Jason O’Connor and Mariusz Papp, all authors are employees of Johnson & Johnson. The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

