Pharmacokinetics of cannabidiol administered by 3 delivery methods at 2 different dosages to healthy dogs
- PMID: 30026641
- PMCID: PMC6038832
Pharmacokinetics of cannabidiol administered by 3 delivery methods at 2 different dosages to healthy dogs
Abstract
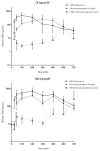
The purpose of this study was to determine the pharmacokinetics of cannabidiol (CBD) in healthy dogs. Thirty, healthy research dogs were assigned to receive 1 of 3 formulations (oral microencapsulated oil beads, oral CBD-infused oil, or CBD-infused transdermal cream), at a dose of 75 mg or 150 mg q12h for 6 wk. Serial cannabidiol plasma concentrations were measured over the first 12 h and repeated at 2, 4, and 6 wk. Higher systemic exposures were observed with the oral CBD-infused oil formulation and the half-life after a 75-mg and 150-mg dose was 199.7 ± 55.9 and 127.5 ± 32.2 min, respectively. Exposure is dose-proportional and the oral CBD-infused oil provides the most favorable pharmacokinetic profile.
Le but de la présente étude était de déterminer la pharmacocinétique du cannbidiol (CBD) chez des chiens en santé. Trente chiens de recherche en santé ont été assignés à recevoir une des trois formulations (de l’huile micro-encapsulé dans des billes par voie orale, de l’huile infusé de CBD par voie orale, ou une crème infusée de CBD par voie transdermique), à une dose de 75 mg ou 150 mg q12h pendant 6 semaines. Les concentrations plasmatiques de cannabidiol ont été mesurées pendant les 12 premières heures et répétées après 2, 4 et 6 semaines. Les expositions systémiques les plus élevées ont été observées avec la formulation d’huile infusé de CBD administrée par voie orale et la demi-vie après une dose de 75 mg et de 150 mg était de 199,7 ± 55,9 et 127,5 ± 32,2 min, respectivement. L’exposition est proportionnelle à la dose et l’huile infusée de CBD par voie orale fournie le profile pharmacocinétique le plus favorable.(Traduit par Docteur Serge Messier).
Figures

References
-
- Ben Amar M. Cannabinoids in medicine: A review of their therapeutic potential. J Ethnopharmacol. 2006;105:1–25. - PubMed
-
- Abel EL. Marihuana, The First Twelve Thousand Years. New York, New York: Plenum Press; 1980.
-
- Russo E, Guy GW. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses. 2006;66:234–246. - PubMed
-
- Robson PJ. Therapeutic potential of cannabinoid medicines. Drug Test Anal. 2014;6:24–30. - PubMed
-
- Podell M. Highs and lows of medical marijuana in the treatment of epilepsy. Proc Am Coll Vet Intern Med Forum. 2015:331–333.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
