Powerful anti-colon cancer effect of modified nanoparticle-mediated IL-15 immunogene therapy through activation of the host immune system
- PMID: 30026861
- PMCID: PMC6037032
- DOI: 10.7150/thno.24157
Powerful anti-colon cancer effect of modified nanoparticle-mediated IL-15 immunogene therapy through activation of the host immune system
Erratum in
-
Erratum: Powerful anti-colon cancer effect of modified nanoparticle-mediated IL-15 immunogene therapy through activation of the host immune system: Erratum.Theranostics. 2019 Aug 17;9(22):6466-6467. doi: 10.7150/thno.39082. eCollection 2019. Theranostics. 2019. PMID: 31588229 Free PMC article.
Abstract
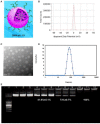
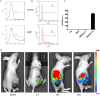
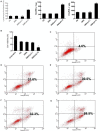
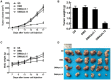
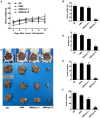
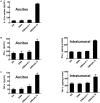
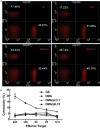
Rationale: Colorectal cancer (CRC) is the third most commonly diagnosed cancer around the world. Over the past several years, immunotherapy has demonstrated considerable clinical benefit in CRC therapy, and the number of immunologic therapies for cancer treatment continues to climb each year. Interleukin-15 (IL15), a potent pro-inflammatory cytokine, has emerged as a candidate immunomodulator for the treatment of CRC. Methods: In this study, we developed a novel gene delivery system with a self-assembly method using DOTAP and MPEG-PLA (DMA) to carry pIL15, denoted as DMA-pIL15 which was used to treat tumor-bearing mice. Results: Supernatant from lymphocytes treated with supernatant derived from CT26 cells transfected with DMA-pIL15 inhibited the growth of CT26 cells and induced cell apoptosis in vitro. Treatment of tumor-bearing mice with DMA-pIL15 complex significantly inhibited tumor growth in both subcutaneous and peritoneal models in vivo by inhibiting angiogenesis, promoting apoptosis, and reducing proliferation through activation of the host immune system. Conclusion: The IL-15 plasmid and DMA complex showed promise for treating CRC clinically as an experimental new drug.
Keywords: DOTAP; MPEG-PLA; colorectal cancer; immunotherapy; interleukin-15.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Torre LA, Bray F, Siegel RL. et al. Global cancer statistics. CA Cancer J Clin. 2015;65:87–108. - PubMed
-
- Mongan JP, Fadul CE, Cole BF. et al. Brain metastases from colorectal cancer: risk factors, incidence, and the possible role of chemokines. Clin Colorectal Cancer. 2009;8:100–5. - PubMed
-
- Shah MA, Renfro LA, Allegra CJ. et al. Impact of patient factors on recurrence risk and time dependency of oxaliplatin benefit in patients with colon cancer: analysis from modern-era adjuvant studies in the adjuvant colon cancer end points (ACCENT) database. J Clin Oncol. 2016;34:843–53. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

