Effect of the estrus cycle stage on the establishment of murine endometriosis lesions
- PMID: 30027146
- PMCID: PMC6046203
Effect of the estrus cycle stage on the establishment of murine endometriosis lesions
Abstract
Background: Establishment of a standardized animal endometriosis model is necessary for evaluation of new drug effects and for explaining different ethological aspects of this disease. For this purpose, we need a model which has more similarity to human endometriosis.
Objective: Our objective was to establish an autologous endometriosis mouse model based on endogenous estrogen level and analyze the influence of estrus cycle on the maintenance of endometriotic lesions.
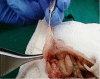
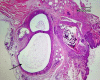
Materials and methods: In this experimental study, endometriotic lesions were induced in 52 female NMRI mice by suturing uterine tissue samples to the abdominal wall. The transplantation was either performed at proestrus/estrus or at metestrus/diestrus cycles. Urine-soaked beddings from males and also male vasectomized mice were transferred to the cages to synchronize and maintenance of estrus cycle in female mice. The mice were sacrificed after different transplantation periods (2, 4, 6 or 8 wk). The lesions size, macroscopic growth, model success rate, histological and immune-histochemical analyses were assessed at the end.
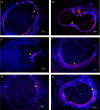
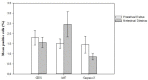
Results: From a total of 200 tissue samples sutured into the peritoneal cavity, 83 endometriotic lesions were confirmed by histopathology (41.5%). Model success rate for proestrus/estrus mice was 60.7% vs. 79.2% for metestrus/diestrus mice. The endometriotic lesions had similar growth in both groups. Number of caspase-3, Ki67-positive cells and CD31-positive micro vessels were also similar in endometriotic lesions of two groups.
Conclusion: If we maintain the endogenous estrogen levels in mice, we can induce endometriosis mouse model in both proestrus/estrus and metestrus/diestrus cycle without any significant difference.
Keywords: Animal models; Autologous; Endometriosis; Estrus cycle; Transplantation.
Conflict of interest statement
None Declared.
Figures

References
-
- Grummer R. Animal models in endometriosis research. Hum Reprod Update. 2006;12:641–649. - PubMed
-
- Story L, Kennedy S. Animal studies in endometriosis: a review. ILAR J. 2004;45:132–138. - PubMed
-
- do Amaral VF, Dal Lago EA, Kondo W, Souza LC, Francisco JC. Development of an experimental model of endometriosis in rats. Rev Col Bras Cir. 2009;36:250–255. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
