Antimicrobial Resistance in Bordetella bronchiseptica
- PMID: 30027886
- PMCID: PMC11633599
- DOI: 10.1128/microbiolspec.ARBA-0024-2017
Antimicrobial Resistance in Bordetella bronchiseptica
Abstract
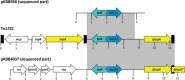
Bordetella bronchiseptica is involved in respiratory tract infections mainly in dogs and pigs but may also cause infections in humans. Valid and representative data on antimicrobial susceptibility of B. bronchiseptica is rare. Approved antimicrobial susceptibility testing methods have been published, but very few clinical breakpoints are available. The MIC values are low for most agents but high for β-lactam antibiotics and macrolides. Information on the genetic basis of resistance is scarce. For a small number of isolates that are resistant or show elevated MICs, the molecular basis of resistance was identified. Three tetracycline resistance genes, tet(A), tet(C), and tet(31), coding for major facilitator superfamily efflux pumps, were identified. Two other major facilitator superfamily exporter genes confer resistance to chloramphenicol (cmlB1) or to chloramphenicol and florfenicol (floR). Two class B chloramphenicol acetyltransferase genes (catB1 and catB3), which confer resistance to nonfluorinated phenicols by enzymatic inactivation, have been identified in B. bronchiseptica. Like the trimethoprim resistance genes dfrA1 and dfrB1, which code for trimethoprim-insensitive dihydrofolate reductases, the genes catB1 and catB3 were located on gene cassettes and found in class 1 integrons also harboring the sulfonamide resistance gene sul1. In addition, the gene sul2 has also been detected. Both sul1 and sul2 code for sulfonamide-insensitive dihydropteroate synthases. A gene cassette harboring the β-lactamase gene blaOXA-2 was also identified, whereas β-lactam resistance in B. bronchiseptica seems to be more likely due to reduced influx in combination with the species-specific β-lactamase encoded by blaBOR-1. The resistance genes were mostly located on conjugative plasmids.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

