Sorafenib Combined with 5-azacytidine in Older Patients with Untreated FLT3-ITD Mutated Acute Myeloid Leukemia
- PMID: 30028037
- PMCID: PMC12422155
- DOI: 10.1002/ajh.25198
Sorafenib Combined with 5-azacytidine in Older Patients with Untreated FLT3-ITD Mutated Acute Myeloid Leukemia
Abstract
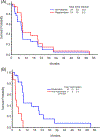
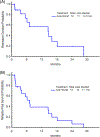
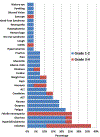
Based on our previous study of the combination of sorafenib with 5-azacytidine (AZA) in relapsed/refractory patients with FLT3 mutated acute myeloid leukemia (AML), we hypothesized that the combination would be efficacious and well tolerated in untreated patients with FLT3 mutated AML who are unsuitable for standard chemotherapy due to advanced age or lack of fitness. Newly diagnosed patients with untreated FLT3 mutated AML who underwent frontline therapy on 2 separate protocols of AZA plus sorafenib were analyzed. The clinical trials were registered at clinicaltrials.gov (NCT02196857 and NCT01254890). Overall, 27 patients with untreated FLT3 mutated AML (median age of 74 years, range, 61-86) were enrolled. The overall response rate was 78% (7 [26%] CR, 12 [44%] CRi/CRp, and 2 [7%] PR). Patients received a median of 3 treatment cycles (1-35). The median duration of CR/CRp/CRi is 14.5 months (1.1-28.7 months). Three (11%) responding patients (1 CR, 2 CRi) proceeded to allogeneic stem cell transplant. The median follow-up for surviving patients was 4.1 months (3.0-17.3 months). The median overall survival for the entire group was 8.3 months, and 9.2 months in the 19 responders. The regimen was well tolerated in elderly patients with untreated FLT3 mutated AML with no early deaths.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest with the contents of this article.
Figures
References
-
- Cheson BD, Cassileth PA, Head DR, et al. Report of the National Cancer Institute-sponsored workshop on definitions of diagnosis and response in acute myeloid leukemia. J Clin Oncol. 1990;8:813–819. - PubMed
-
- Whitman SP, Maharry K, Radmacher MD, et al. FLT3 internal tandem duplication associates with adverse outcome and gene- and microRNA-expression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. 2010;116:3622–3626. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
