Caveolin-3 KO disrupts t-tubule structure and decreases t-tubular ICa density in mouse ventricular myocytes
- PMID: 30028203
- PMCID: PMC6415741
- DOI: 10.1152/ajpheart.00209.2018
Caveolin-3 KO disrupts t-tubule structure and decreases t-tubular ICa density in mouse ventricular myocytes
Abstract
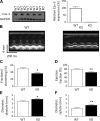
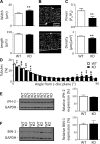
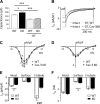
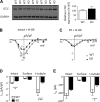
Caveolin-3 (Cav-3) is a protein that has been implicated in t-tubule formation and function in cardiac ventricular myocytes. In cardiac hypertrophy and failure, Cav-3 expression decreases, t-tubule structure is disrupted, and excitation-contraction coupling is impaired. However, the extent to which the decrease in Cav-3 expression underlies these changes is unclear. We therefore investigated the structure and function of myocytes isolated from the hearts of Cav-3 knockout (KO) mice. These mice showed cardiac dilatation and decreased ejection fraction in vivo compared with wild-type control mice. Isolated KO myocytes showed cellular hypertrophy, altered t-tubule structure, and decreased L-type Ca2+ channel current ( ICa) density. This decrease in density occurred predominantly in the t-tubules, with no change in total ICa, and was therefore a consequence of the increase in membrane area. Cav-3 KO had no effect on L-type Ca2+ channel expression, and C3SD peptide, which mimics the scaffolding domain of Cav-3, had no effect on ICa in KO myocytes. However, inhibition of PKA using H-89 decreased ICa at the surface and t-tubule membranes in both KO and wild-type myocytes. Cav-3 KO had no significant effect on Na+/Ca2+ exchanger current or Ca2+ release. These data suggest that Cav-3 KO causes cellular hypertrophy, thereby decreasing t-tubular ICa density. NEW & NOTEWORTHY Caveolin-3 (Cav-3) is a protein that inhibits hypertrophic pathways, has been implicated in the formation and function of cardiac t-tubules, and shows decreased expression in heart failure. This study demonstrates that Cav-3 knockout mice show cardiac dysfunction in vivo, while isolated ventricular myocytes show cellular hypertrophy, changes in t-tubule structure, and decreased t-tubular L-type Ca2+ current density, suggesting that decreased Cav-3 expression contributes to these changes in cardiac hypertrophy and failure.
Keywords: calcium current; calcium release; calcium transient; caveolin-3.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
-
- Augustus AS, Buchanan J, Addya S, Rengo G, Pestell RG, Fortina P, Koch WJ, Bensadoun A, Abel ED, Lisanti MP. Substrate uptake and metabolism are preserved in hypertrophic caveolin-3 knockout hearts. Am J Physiol Heart Circ Physiol 295: H657–H666, 2008. doi:10.1152/ajpheart.00387.2008. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

