Engineering a Rugged Nanoscaffold To Enhance Plug-and-Display Vaccination
- PMID: 30028591
- PMCID: PMC6158681
- DOI: 10.1021/acsnano.8b02805
Engineering a Rugged Nanoscaffold To Enhance Plug-and-Display Vaccination
Abstract
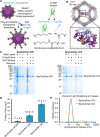
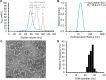
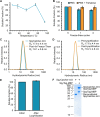
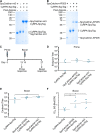
Nanoscale organization is crucial to stimulating an immune response. Using self-assembling proteins as multimerization platforms provides a safe and immunogenic system to vaccinate against otherwise weakly immunogenic antigens. Such multimerization platforms are generally based on icosahedral viruses and have led to vaccines given to millions of people. It is unclear whether synthetic protein nanoassemblies would show similar potency. Here we take the computationally designed porous dodecahedral i301 60-mer and rationally engineer this particle, giving a mutated i301 (mi3) with improved particle uniformity and stability. To simplify the conjugation of this nanoparticle, we employ a SpyCatcher fusion of mi3, such that an antigen of interest linked to the SpyTag peptide can spontaneously couple through isopeptide bond formation (Plug-and-Display). SpyCatcher-mi3 expressed solubly to high yields in Escherichia coli, giving more than 10-fold greater yield than a comparable phage-derived icosahedral nanoparticle, SpyCatcher-AP205. SpyCatcher-mi3 nanoparticles showed high stability to temperature, freeze-thaw, lyophilization, and storage over time. We demonstrate approximately 95% efficiency coupling to different transmission-blocking and blood-stage malaria antigens. Plasmodium falciparum CyRPA was conjugated to SpyCatcher-mi3 nanoparticles and elicited a high avidity antibody response, comparable to phage-derived virus-like particles despite their higher valency and RNA cargo. The simple production, precise derivatization, and exceptional ruggedness of this nanoscaffold should facilitate broad application for nanobiotechnology and vaccine development.
Keywords: bioconjugation; bionanotechnology; nanomedicine; protein engineering; self-assembly; vaccination; virus-like particle.
Conflict of interest statement
The authors declare the following competing financial interest(s): M.H. is an inventor on a patent regarding peptide targeting
Figures

References
-
- Mitragotri S.; Anderson D. G.; Chen X.; Chow E. K.; Ho D.; Kabanov A. V.; Karp J. M.; Kataoka K.; Mirkin C. A.; Petrosko S. H.; Shi J.; Stevens M. M.; Sun S.; Teoh S.; Venkatraman S. S.; Xia Y.; Wang S.; Gu Z.; Xu C. Accelerating the Translation of Nanomaterials in Biomedicine. ACS Nano 2015, 9, 6644–6654. 10.1021/acsnano.5b03569. - DOI - PMC - PubMed
-
- Uchida M.; McCoy K.; Fukuto M.; Yang L.; Yoshimura H.; Miettinen H. M.; LaFrance B.; Patterson D. P.; Schwarz B.; Karty J. A.; Prevelige P. E.; Lee B.; Douglas T. Modular Self-Assembly of Protein Cage Lattices for Multistep Catalysis. ACS Nano 2018, 12, 942–953. 10.1021/acsnano.7b06049. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

