Abiraterone Alone or in Combination With Enzalutamide in Metastatic Castration-Resistant Prostate Cancer With Rising Prostate-Specific Antigen During Enzalutamide Treatment
- PMID: 30028657
- PMCID: PMC6118405
- DOI: 10.1200/JCO.2018.77.9827
Abiraterone Alone or in Combination With Enzalutamide in Metastatic Castration-Resistant Prostate Cancer With Rising Prostate-Specific Antigen During Enzalutamide Treatment
Abstract
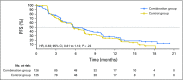
Purpose Enzalutamide resistance could result from raised androgens and be overcome by combination with abiraterone acetate. PLATO ( ClinicalTrials.gov identifier: NCT01995513) interrogated this hypothesis using a randomized, double-blind, placebo-controlled design. Patients and Methods In period one, men with chemotherapy-naïve metastatic castration-resistant prostate cancer received open-label enzalutamide 160 mg daily. Men with no prostate-specific antigen (PSA) increase at weeks 13 and 21 were treated until PSA progression (≥ 25% increase and ≥ 2 ng/mL above nadir), then randomly assigned at a one-to-one ratio in period two to abiraterone acetate 1,000 mg daily and prednisone 5 mg twice daily with either enzalutamide or placebo (combination or control group, respectively) until disease progression as defined by the primary end point: progression-free survival (radiographic or unequivocal clinical progression or death during study). Secondary end points included time to PSA progression and PSA response in period two. Results Of 509 patients enrolled in period one, 251 were randomly assigned in period two. Median progression-free survival was 5.7 months in the combination group and 5.6 months in the control group (hazard ratio, 0.83; 95% CI, 0.61 to 1.12; P = .22). There was no difference in the secondary end points. Grade 3 hypertension (10% v 2%) and increased ALT (6% v 2%) or AST (2% v 0%) were more frequent in the combination than the control group. Conclusion Combining enzalutamide with abiraterone acetate and prednisone is not indicated after PSA progression during treatment with enzalutamide alone; hypertension and elevated liver enzymes are more frequent with combination therapy.
Figures



References
-
- Ryan CJ, Smith MR, Fizazi K, et al. : Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): Final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 16:152-160, 2015 - PubMed
-
- Attard G, Cooper CS, de Bono JS: Steroid hormone receptors in prostate cancer: A hard habit to break? Cancer Cell 16:458-462, 2009 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

