Safety monitoring of ROTAVAC vaccine and etiological investigation of intussusception in India: study protocol
- PMID: 30029630
- PMCID: PMC6053826
- DOI: 10.1186/s12889-018-5809-7
Safety monitoring of ROTAVAC vaccine and etiological investigation of intussusception in India: study protocol
Abstract
Background: ROTAVAC, an indigenous rotavirus vaccine, was introduced in the universal immunization program of India in four states in 2016 and expanded to five more states in 2017. The clinical trial on efficacy of ROTAVAC did not detect an increased risk of intussusception, but the trial was not large enough to detect a small risk. This protocol paper describes the establishment and implementation of a surveillance system to monitor the safety of rotavirus vaccine and investigate the potential infectious etiologies of intussusception.
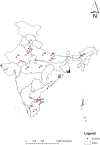
Methods: This is a multi-centric hospital-based active surveillance being conducted at 28 hospitals in nine states of India. Data gathered from surveillance will be used to assess the risk of intussusception after ROTAVAC administration and to determine the infectious etiologies of intussusception. For safety assessment of ROTAVAC vaccine, children aged less than two years with intussusception admitted at the sentinel hospitals are enrolled into surveillance, a case report form completed, and a copy of the vaccination card obtained. The risk of intussusception following rotavirus vaccination will be assessed using a self-controlled case-series design. The investigation for potential infectious etiologies of intussusception is through a matched case-control design. Children enrolled for the safety assessment serve as cases and for each case, an age, gender and location matched control is enrolled within 30 days of case enrollment. Stool specimens are obtained from cases and controls. All forms and specimens are sent to the referral laboratory for data entry, analysis, multiplexed molecular testing, and storage.
Discussion: Anticipated public health benefits of this surveillance include the generation of information useful to national government on safety of vaccine and to make future decisions on vaccine use through risk-benefit analysis. Investigating infectious agents may help to determine the potential infectious etiologies of intussusception.
Keywords: India; Infectious etiologies; Intussusception; ROTAVAC; Rotavirus vaccine; Self-controlled case series methods.
Conflict of interest statement
Consent for publication
Not applicable
Competing interests
Venkata Raghava Mohan is an Associate Editor for BMC Public Health. The other authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- WHO Rotavirus vaccines. WHO position paper – January 2013. Wkly Epidemiol Rec. 2013;88:49–64. - PubMed
-
- Shri JP Nadda launches Rotavirus vaccine as part of Universal Immunization Programme; terms it a “historic moment” [Internet]. Available from: http://pib.nic.in/newsite/PrintRelease.aspx?relid=138342 [cited 2018 Jun 17].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

