Simultaneous Improvements of Pseudomonas Cell Growth and Polyhydroxyalkanoate Production from a Lignin Derivative for Lignin-Consolidated Bioprocessing
- PMID: 30030226
- PMCID: PMC6121988
- DOI: 10.1128/AEM.01469-18
Simultaneous Improvements of Pseudomonas Cell Growth and Polyhydroxyalkanoate Production from a Lignin Derivative for Lignin-Consolidated Bioprocessing
Abstract
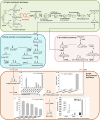
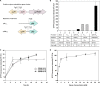
Cell growth and polyhydroxyalkanoate (PHA) biosynthesis are two key traits in PHA production from lignin or its derivatives. However, the links between them remain poorly understood. Here, the transcription levels of key genes involved in PHA biosynthesis were tracked in Pseudomonas putida strain A514 grown on vanillic acid as the sole carbon source under different levels of nutrient availability. First, enoyl-coenzyme A (CoA) hydratase (encoded by phaJ4) is stress induced and likely to contribute to PHA synthesis under nitrogen starvation conditions. Second, much higher expression levels of 3-hydroxyacyl-acyl carrier protein (ACP) thioesterase (encoded by phaG) and long-chain fatty acid-CoA ligase (encoded by alkK) under both high and low nitrogen (N) led to the hypothesis that they likely not only have a role in PHA biosynthesis but are also essential to cell growth. Third, 40 mg/liter PHA was synthesized by strain AphaJ4C1 (overexpression of phaJ4 and phaC1 in strain A514) under low-N conditions, in contrast to 23 mg/liter PHA synthesized under high-N conditions. Under high-N conditions, strain AalkKphaGC1 (overexpression of phaG, alkK, and phaC1 in A514) produced 90 mg/liter PHA with a cell dry weight of 667 mg/liter, experimentally validating our hypothesis. Finally, further enhancement in cell growth (714 mg/liter) and PHA titer (246 mg/liter) was achieved in strain Axyl_alkKphaGC1 via transcription level optimization, which was regulated by an inducible strong promoter with its regulator, XylR-PxylA, from the xylose catabolic gene cluster of the A514 genome. This study reveals genetic features of genes involved in PHA synthesis from a lignin derivative and provides a novel strategy for rational engineering of these two traits, laying the foundation for lignin-consolidated bioprocessing.IMPORTANCE With the recent advances in processing carbohydrates in lignocellulosics for bioproducts, almost all biological conversion platforms result in the formation of a significant amount of lignin by-products, representing the second most abundant feedstock on earth. However, this resource is greatly underutilized due to its heterogeneity and recalcitrant chemical structure. Thus, exploiting lignin valorization routes would achieve the complete utilization of lignocellulosic biomass and improve cost-effectiveness. The culture conditions that encourage cell growth and polyhydroxyalkanoate (PHA) accumulation are different. Such an inconsistency represents a major hurdle in lignin-to-PHA bioconversion. In this study, we traced and compared transcription levels of key genes involved in PHA biosynthesis pathways in Pseudomonas putida A514 under different nitrogen concentrations to unveil the unusual features of PHA synthesis. Furthermore, an inducible strong promoter was identified. Thus, the molecular features and new genetic tools reveal a strategy to coenhance PHA production and cell growth from a lignin derivative.
Keywords: Pseudomonas putida; lignin-consolidated bioprocessing; polyhydroxyalkanoate synthesis.
Copyright © 2018 American Society for Microbiology.
Figures
References
-
- Linger JG, Vardon DR, Guarnieri MT, Karp EM, Hunsinger GB, Franden MA, Johnson CW, Chupka G, Strathmann TJ, Pienkos PT, Beckham GT. 2014. Lignin valorization through integrated biological funneling and chemical catalysis. Proc Natl Acad Sci U S A 111:12013–12018. doi:10.1073/pnas.1410657111. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

