Myeloid Dendritic Cells Induce HIV Latency in Proliferating CD4+ T Cells
- PMID: 30030324
- PMCID: PMC6103814
- DOI: 10.4049/jimmunol.1701233
Myeloid Dendritic Cells Induce HIV Latency in Proliferating CD4+ T Cells
Abstract
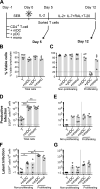
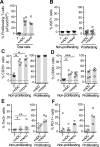
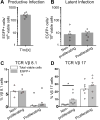
HIV latency occurs predominantly in long-lived resting CD4+ T cells; however, latent infection also occurs in T cell subsets, including proliferating CD4+ T cells. We compared the establishment and maintenance of latent infection in nonproliferating and proliferating human CD4+ T cells cocultured with syngeneic myeloid dendritic cells (mDC). Resting CD4+ T cells were labeled with the proliferation dye eFluor 670 and cultured alone or with mDC, plasmacytoid dendritic cells, or monocytes in the presence of staphylococcal enterotoxin B (SEB). Cells were cultured for 24 h and infected with CCR5-tropic enhanced GFP (EGFP) reporter HIV. Five days postinfection, nonproductively infected EGFP- CD4+ T cells that were either nonproliferating (eFluor 670hi) or proliferating (eFluor 670lo) were sorted and cultured for an additional 7 d (day 12) with IL-7 and antiretrovirals. At day 5 postinfection, sorted, nonproductively infected T cells were stimulated with anti-CD3/CD28, and induced expression of EGFP was measured to determine the frequency of latent infection. Integrated HIV in these cells was confirmed using quantitative PCR. By these criteria, latent infection was detected at day 5 and 12 in proliferating T cells cocultured with mDC and monocytes but not plasmacytoid dendritic cells, where CD4+ T cells at day 12 were poor. At day 5 postinfection, nonproliferating T cells expressing SEB-specific TCR Vβ-17 were enriched in latent infection compared with non-SEB-specific TCR Vβ-8.1. Together, these data show that both nonproliferating and proliferating CD4+ T cells can harbor latent infection during SEB-stimulated T cell proliferation and that the establishment of HIV latency in nonproliferating T cells is linked to expression of specific TCR that respond to SEB.
Copyright © 2018 by The American Association of Immunologists, Inc.
Conflict of interest statement
Authors have no conflict of interest to declare.
Figures


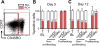
References
-
- Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–90. - PubMed
-
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–300. - PubMed
-
- Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–8. - PubMed
-
- Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, Boucher G, Boulassel MR, Ghattas G, Brenchley JM, Schacker TW, Hill BJ, Douek DC, Routy JP, Haddad EK, Sekaly RP. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

