Mitochondrial dynamics: overview of molecular mechanisms
- PMID: 30030364
- PMCID: PMC6056715
- DOI: 10.1042/EBC20170104
Mitochondrial dynamics: overview of molecular mechanisms
Abstract
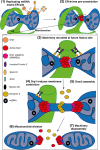
Mitochondria are highly dynamic organelles undergoing coordinated cycles of fission and fusion, referred as 'mitochondrial dynamics', in order to maintain their shape, distribution and size. Their transient and rapid morphological adaptations are crucial for many cellular processes such as cell cycle, immunity, apoptosis and mitochondrial quality control. Mutations in the core machinery components and defects in mitochondrial dynamics have been associated with numerous human diseases. These dynamic transitions are mainly ensured by large GTPases belonging to the Dynamin family. Mitochondrial fission is a multi-step process allowing the division of one mitochondrion in two daughter mitochondria. It is regulated by the recruitment of the GTPase Dynamin-related protein 1 (Drp1) by adaptors at actin- and endoplasmic reticulum-mediated mitochondrial constriction sites. Drp1 oligomerization followed by mitochondrial constriction leads to the recruitment of Dynamin 2 to terminate membrane scission. Inner mitochondrial membrane constriction has been proposed to be an independent process regulated by calcium influx. Mitochondrial fusion is driven by a two-step process with the outer mitochondrial membrane fusion mediated by mitofusins 1 and 2 followed by inner membrane fusion, mediated by optic atrophy 1. In addition to the role of membrane lipid composition, several members of the machinery can undergo post-translational modifications modulating these processes. Understanding the molecular mechanisms controlling mitochondrial dynamics is crucial to decipher how mitochondrial shape meets the function and to increase the knowledge on the molecular basis of diseases associated with morphology defects. This article will describe an overview of the molecular mechanisms that govern mitochondrial fission and fusion in mammals.
Keywords: Dynamin family; ER-Actin; Mitochondrial dynamics; Molecular Mechanisms; Regulation.
© 2018 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

