Mouse modeling and structural analysis of the p.G307S mutation in human cystathionine β-synthase (CBS) reveal effects on CBS activity but not stability
- PMID: 30030379
- PMCID: PMC6130948
- DOI: 10.1074/jbc.RA118.002164
Mouse modeling and structural analysis of the p.G307S mutation in human cystathionine β-synthase (CBS) reveal effects on CBS activity but not stability
Abstract
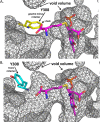
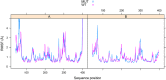
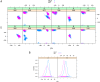
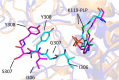
Mutations in the cystathionine β-synthase (CBS) gene are the cause of classical homocystinuria, the most common inborn error in sulfur metabolism. The p.G307S mutation is the most frequent cause of CBS deficiency in Ireland, which has the highest prevalence of CBS deficiency in Europe. Individuals homozygous for this mutation tend to be severely affected and are pyridoxine nonresponsive, but the molecular basis for the strong effects of this mutation is unclear. Here, we characterized a transgenic mouse model lacking endogenous Cbs and expressing human p.G307S CBS protein from a zinc-inducible metallothionein promoter (Tg-G307S Cbs-/-). Unlike mice expressing other mutant CBS alleles, the Tg-G307S transgene could not efficiently rescue neonatal lethality of Cbs-/- in a C57BL/6J background. In a C3H/HeJ background, zinc-induced Tg-G307S Cbs-/- mice expressed high levels of p.G307S CBS in the liver, and this protein variant forms multimers, similarly to mice expressing WT human CBS. However, the p.G307S enzyme had no detectable residual activity. Moreover, treating mice with proteasome inhibitors failed to significantly increase CBS-specific activity. These findings indicated that the G307S substitution likely affects catalytic function as opposed to causing a folding defect. Using molecular dynamics simulation techniques, we found that the G307S substitution likely impairs catalytic function by limiting the ability of the tyrosine at position 308 to assume the proper conformational state(s) required for the formation of the pyridoxal-cystathionine intermediate. These results indicate that the p.G307S CBS is stable but enzymatically inert and therefore unlikely to respond to chaperone-based therapy.
Keywords: bortezomib; cystathionine β-synthase; homocystinuria; inborn error of metabolism; methionine; missense mutation; mouse genetics; p.G307S; pyridoxal phosphate; pyridoxine; structural model; sulfur metabolism; transsulfuration pathway.
© 2018 Gupta et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures




References
-
- Yap S., and Naughten E. (1998) Homocystinuria due to cystathionine β-synthase deficiency in Ireland: 25 years' experience of a newborn screened and treated population with reference to clinical outcome and biochemical control. J. Inherit. Metab. Dis. 21, 738–747 10.1023/A:1005445132327 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

