3D Printed Microfluidic Probes
- PMID: 30030464
- PMCID: PMC6054653
- DOI: 10.1038/s41598-018-29304-x
3D Printed Microfluidic Probes
Abstract
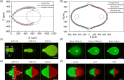
In this work, we fabricate microfluidic probes (MFPs) in a single step by stereolithographic 3D printing and benchmark their performance with standard MFPs fabricated via glass or silicon micromachining. Two research teams join forces to introduce two independent designs and fabrication protocols, using different equipment. Both strategies adopted are inexpensive and simple (they only require a stereolithography printer) and are highly customizable. Flow characterization is performed by reproducing previously published microfluidic dipolar and microfluidic quadrupolar reagent delivery profiles which are compared to the expected results from numerical simulations and scaling laws. Results show that, for most MFP applications, printer resolution artifacts have negligible impact on probe operation, reagent pattern formation, and cell staining results. Thus, any research group with a moderate resolution (≤100 µm) stereolithography printer will be able to fabricate the MFPs and use them for processing cells, or generating microfluidic concentration gradients. MFP fabrication involved glass and/or silicon micromachining, or polymer micromolding, in every previously published article on the topic. We therefore believe that 3D printed MFPs is poised to democratize this technology. We contribute to initiate this trend by making our CAD files available for the readers to test our "print & probe" approach using their own stereolithographic 3D printers.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Ainla A, Jeffries G, Jesorka A. Hydrodynamic flow confinement technology in microfluidic perfusion devices. Micromachines. 2012;3(2):442–461. doi: 10.3390/mi3020442. - DOI
-
- Quate C. Scanning probes as a lithography tool for nanostructures. Surface Science. 1997;386(1–3):259–264. doi: 10.1016/S0039-6028(97)00305-1. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

