Neurodegeneration in diabetic retinopathy: does it really matter?
- PMID: 30030554
- PMCID: PMC6096638
- DOI: 10.1007/s00125-018-4692-1
Neurodegeneration in diabetic retinopathy: does it really matter?
Abstract
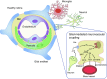
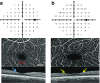
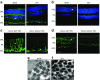
The concept of diabetic retinopathy as a microvascular disease has evolved, in that it is now considered a more complex diabetic complication in which neurodegeneration plays a significant role. In this article we provide a critical overview of the role of microvascular abnormalities and neurodegeneration in the pathogenesis of diabetic retinopathy. A special emphasis is placed on the pathophysiology of the neurovascular unit (NVU), including the contributions of microvascular and neural elements. The potential mechanisms linking retinal neurodegeneration and early microvascular impairment, and the effects of neuroprotective drugs are summarised. Additionally, we discuss how the assessment of retinal neurodegeneration could be an important index of cognitive status, thus helping to identify individuals at risk of dementia, which will impact on current procedures for diabetes management. We conclude that glial, neural and microvascular dysfunction are interdependent and essential for the development of diabetic retinopathy. Despite this intricate relationship, retinal neurodegeneration is a critical endpoint and neuroprotection, itself, can be considered a therapeutic target, independently of its potential impact on microvascular disease. In addition, interventional studies targeting pathogenic pathways that impact the NVU are needed. Findings from these studies will be crucial, not only for increasing our understanding of diabetic retinopathy, but also to help to implement a timely and efficient personalised medicine approach for treating this diabetic complication.
Keywords: Diabetic retinopathy; Microvascular impairment; Neurodegeneration; Neuroprotection; Neurovascular unit; Personalised medicine; Review.
Conflict of interest statement
The authors declare that there is no duality of interest associated with this manuscript.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

