Co-occurrence among three divergent plant-castrating fungi in the same Silene host species
- PMID: 30030861
- PMCID: PMC6340787
- DOI: 10.1111/mec.14805
Co-occurrence among three divergent plant-castrating fungi in the same Silene host species
Abstract
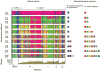
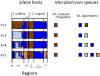
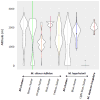
The competitive exclusion principle postulates that different species can only coexist in sympatry if they occupy distinct ecological niches. The goal of this study was to understand the geographical distribution of three species of Microbotryum anther-smut fungi that are distantly related but infect the same host plants, the sister species Silene vulgaris and S. uniflora, in Western Europe. We used microsatellite markers to investigate pathogen distribution in relation to host specialization and ecological factors. Microbotryum violaceo-irregulare was only found on S. vulgaris at high elevations in the Alps. Microbotryum lagerheimii could be subdivided into two genetically differentiated clusters, one on S. uniflora in the UK and the second on S. vulgaris in the Alps and Pyrenees. The most abundant pathogen species, M. silenes-inflatae, could be subdivided into four genetic clusters, co-occurring in the Alps, the UK and the Pyrenees, and was found on both S. vulgaris and S. uniflora. All three fungal species had high levels of homozygosity, in agreement with the selfing mating system generally observed in anther-smut fungi. The three pathogen species and genetic clusters had large range overlaps, but occurred at sites with different elevations, temperatures and precipitation levels. The three Microbotryum species thus do not appear to be maintained by host specialization or geographic allopatry, but instead may occupy different ecological niches in terms of environmental conditions.
Keywords: Microbotryum violaceum; Silene maritima; altitude; biogeography; endemicity; fungi; hybrid zones; population structure; speciation.
© 2018 John Wiley & Sons Ltd.
Figures


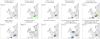
References
-
- Abbate JL, Antonovics J. Elevational disease distribution in a natural plant-pathogen system: insights from changes across host populations and climate. Oikos. 2014;123:1126–1136.
-
- Antonovics J, Hood M, Partain J. The ecology and genetics of a host shift: Microbotryum as a model system. American Naturalist. 2002;160:S40–S53. - PubMed
-
- Arnaud-Haond S, Belkhir K. GENCLONE: a computer program to analyse genotypic data, test for clonality and describe spatial clonal organization. Mol Ecol Notes. 2007;7:15–17.
-
- Badouin H, Gladieux P, Gouzy J, et al. Widespread selective sweeps throughout the genome of model plant pathogenic fungi and identification of effector candidates. Mol Ecol. 2017;26:2041–2062. - PubMed
-
- Berardi AE, Fields PD, Abbate JL, Taylor DR. Elevational divergence and clinal variation in floral color and leaf chemistry in Silene vulgaris. American Journal of Botany. 2016;103:1508–1523. - PubMed
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

