3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes
- PMID: 30032046
- PMCID: PMC6201194
- DOI: 10.1016/j.biomaterials.2018.07.014
3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes
Abstract
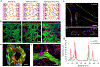
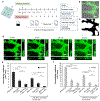
The blood-brain barrier (BBB) regulates molecular trafficking, protects against pathogens, and prevents efficient drug delivery to the brain. Models to date failed to reproduce the human anatomical complexity of brain barriers, contributing to misleading results in clinical trials. To overcome these limitations, a novel 3-dimensional BBB microvascular network model was developed via vasculogenesis to accurately replicate the in vivo neurovascular organization. This microfluidic system includes human induced pluripotent stem cell-derived endothelial cells, brain pericytes, and astrocytes as self-assembled vascular networks in fibrin gel. Gene expression of membrane transporters, tight junction and extracellular matrix proteins, was consistent with computational analysis of geometrical structures and quantitative immunocytochemistry, indicating BBB maturation and microenvironment remodelling. Confocal microscopy validated microvessel-pericyte/astrocyte dynamic contact-interactions. The BBB model exhibited perfusable and selective microvasculature, with permeability lower than conventional in vitro models, and similar to in vivo measurements in rat brain. This robust and physiologically relevant BBB microvascular model offers an innovative and valuable platform for drug discovery to predict neuro-therapeutic transport efficacy in pre-clinical applications as well as recapitulate patient-specific and pathological neurovascular functions in neurodegenerative disease.
Keywords: Drug delivery test platform; Human blood-brain barrier; Induced pluripotent stem cell-derived endothelial cells; In vitro modeling; Microfluidic device; Self-assembled microvascular network.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures





References
-
- Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, and Begley DJ, “Structure and function of the blood-brain barrier,” Neurobiol. Dis, vol. 37, no. 1, pp. 13–25, 2010. - PubMed
-
- Zlokovic BV, “Neurovascular mechanisms of Alzheimer’s neurodegeneration,” Trends Neurosci, vol. 28, no. 4, pp. 202–208, 2005. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

