Subcellular mapping of living cells via synchrotron microFTIR and ZnS hemispheres
- PMID: 30032447
- PMCID: PMC6132686
- DOI: 10.1007/s00216-018-1245-x
Subcellular mapping of living cells via synchrotron microFTIR and ZnS hemispheres
Abstract
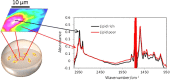
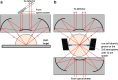
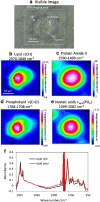
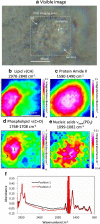
FTIR imaging is a label-free, non-destructive method valuably exploited in the study of the biological process in living cells. However, the long wavelength/low spatial resolution and the strong absorbance of water are still key constrains in the application of IR microscopy ex vivo. In this work, a new retrofit approach based on the use of ZnS hemispheres is introduced to significantly improve the spatial resolution on live cell FTIR imaging. By means of two high refractive index domes sandwiching the sample, a lateral resolution close to 2.2 μm at 6 μm wavelength has been achieved, i.e. below the theoretical diffraction limit in air and more than twice the improvement (to ~λ/2.7) from our previous attempt using CaF2 lenses. The ZnS domes also allowed an extended spectral range to 950 cm-1, in contrast to the cut-off at 1050 cm-1 using CaF2. In combination with synchrotron radiation source, microFTIR provides an improved signal-to-noise ratio through the circa 12 μm thin layer of medium, thus allowing detailed distribution of lipids, protein and nucleic acid in the surround of the nucleus of single living cells. Endoplasmic reticula were clearly shown based on the lipid ν(CH) and ν(C=O) bands, while the DNA was imaged based on the ν(PO2-) band highlighting the nucleus region. This work has also included a demonstration of drug (doxorubicin) in cell measurement to highlight the potential of this approach. Graphical abstract.
Keywords: Anti-cancer drugs; Cell systems/single cell analysis; FT-IR imaging; Fourier transform infrared; High definition; Immersion objective.
Conflict of interest statement
There is no potential conflict of interest from this work.
Figures



References
-
- Alam MK, Timlin JA, Martin LE, Williams D, Lyons CR, Garrison K, et al. Spectroscopic evaluation of living murine macrophage cells before and after activation using attenuated total reflectance infrared spectroscopy. Vib Spectrosc. 2004;34(1):3–11. doi: 10.1016/j.vibspec.2003.07.002. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

