Fish Scales Dictate the Pattern of Adult Skin Innervation and Vascularization
- PMID: 30032992
- PMCID: PMC6561483
- DOI: 10.1016/j.devcel.2018.06.019
Fish Scales Dictate the Pattern of Adult Skin Innervation and Vascularization
Abstract
As animals mature from embryonic to adult stages, the skin grows and acquires specialized appendages, like hairs, feathers, and scales. How cutaneous blood vessels and sensory axons adapt to these dramatic changes is poorly understood. By characterizing skin maturation in zebrafish, we discovered that sensory axons are delivered to the adult epidermis in organized nerves patterned by features in bony scales. These nerves associate with blood vessels and osteoblasts above scales. Osteoblasts create paths in scales that independently guide nerves and blood vessels during both development and regeneration. By preventing scale regeneration and examining mutants lacking scales, we found that scales recruit, organize, and polarize axons and blood vessels to evenly distribute them in the skin. These studies uncover mechanisms for achieving comprehensive innervation and vascularization of the adult skin and suggest that scales coordinate a metamorphosis-like transformation of the skin with sensory axon and vascular remodeling.
Keywords: axon; dorsal root ganglia; metamorphosis; osteoblast; regeneration; scale; sensory nerve; skin; vasculature; zebrafish.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
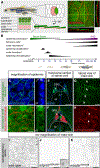
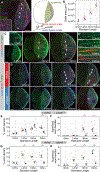



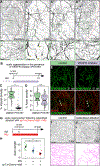

Comment in
-
Scales Radi(i)cally Remodel Sensory Axons and Vasculature.Dev Cell. 2018 Aug 6;46(3):253-254. doi: 10.1016/j.devcel.2018.07.016. Dev Cell. 2018. PMID: 30086298
References
-
- Bostaille N, Gauquier A, Stainier DYR, Raible DW, and Vanhollebeke B (2017). Defective adgra2 (gpR124) splicing and function in zebrafish ouchless mutants. Development 144, 8–11. - PubMed
-
- Braverman IM, and Yen A (1977). Ultrastructure of the human dermal microcirculation. II. The capillary loops of the dermal papillae. J. Invest. Dermatol. 68, 44–52. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

