Reactivation of Dormant Relay Pathways in Injured Spinal Cord by KCC2 Manipulations
- PMID: 30033363
- PMCID: PMC6063786
- DOI: 10.1016/j.cell.2018.06.005
Reactivation of Dormant Relay Pathways in Injured Spinal Cord by KCC2 Manipulations
Erratum in
-
Reactivation of Dormant Relay Pathways in Injured Spinal Cord by KCC2 Manipulations.Cell. 2018 Sep 6;174(6):1599. doi: 10.1016/j.cell.2018.08.050. Cell. 2018. PMID: 30193115 Free PMC article. No abstract available.
Abstract
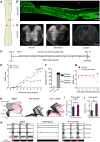
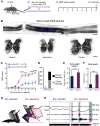
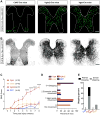
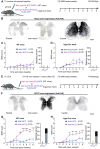
Many human spinal cord injuries are anatomically incomplete but exhibit complete paralysis. It is unknown why spared axons fail to mediate functional recovery in these cases. To investigate this, we undertook a small-molecule screen in mice with staggered bilateral hemisections in which the lumbar spinal cord is deprived of all direct brain-derived innervation, but dormant relay circuits remain. We discovered that a KCC2 agonist restored stepping ability, which could be mimicked by selective expression of KCC2, or hyperpolarizing DREADDs, in the inhibitory interneurons between and around the staggered spinal lesions. Mechanistically, these treatments transformed this injury-induced dysfunctional spinal circuit to a functional state, facilitating the relay of brain-derived commands toward the lumbar spinal cord. Thus, our results identify spinal inhibitory interneurons as a roadblock limiting the integration of descending inputs into relay circuits after injury and suggest KCC2 agonists as promising treatments for promoting functional recovery after spinal cord injury.
Keywords: KCC2; excitability; excitation/inhibition balance; inhibitory neurons; propriospinal pathways; spinal cord injury.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures

Comment in
-
Unsilencing spared spinal neurons.Nat Rev Neurosci. 2018 Sep;19(9):516-517. doi: 10.1038/s41583-018-0047-7. Nat Rev Neurosci. 2018. PMID: 30072756 No abstract available.
-
Reducing neuronal inhibition restores locomotion in paralysed mice.Nature. 2018 Sep;561(7723):317-318. doi: 10.1038/d41586-018-06651-3. Nature. 2018. PMID: 30224731 No abstract available.
References
-
- Arber S. Motor circuits in action: specification, connectivity, and function. Neuron. 2012;74:975–989. - PubMed
-
- Ballermann M, Fouad K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur J Neurosci. 2006;23:1988–1996. - PubMed
-
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. - PubMed
-
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

