A Complete Electron Microscopy Volume of the Brain of Adult Drosophila melanogaster
- PMID: 30033368
- PMCID: PMC6063995
- DOI: 10.1016/j.cell.2018.06.019
A Complete Electron Microscopy Volume of the Brain of Adult Drosophila melanogaster
Abstract
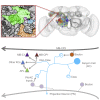
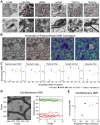
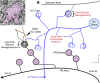
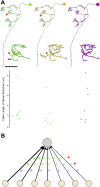
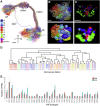
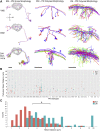
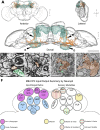
Drosophila melanogaster has a rich repertoire of innate and learned behaviors. Its 100,000-neuron brain is a large but tractable target for comprehensive neural circuit mapping. Only electron microscopy (EM) enables complete, unbiased mapping of synaptic connectivity; however, the fly brain is too large for conventional EM. We developed a custom high-throughput EM platform and imaged the entire brain of an adult female fly at synaptic resolution. To validate the dataset, we traced brain-spanning circuitry involving the mushroom body (MB), which has been extensively studied for its role in learning. All inputs to Kenyon cells (KCs), the intrinsic neurons of the MB, were mapped, revealing a previously unknown cell type, postsynaptic partners of KC dendrites, and unexpected clustering of olfactory projection neurons. These reconstructions show that this freely available EM volume supports mapping of brain-spanning circuits, which will significantly accelerate Drosophila neuroscience. VIDEO ABSTRACT.
Keywords: Drosophila melanogaster; connectomics; electron microscopy; image stitching; mushroom body; neural circuits; olfaction.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures








Comment in
-
The whole fly brain in detail.Nat Methods. 2018 Sep;15(9):651. doi: 10.1038/s41592-018-0125-9. Nat Methods. 2018. PMID: 30171244 No abstract available.
References
-
- Arganda-Carreras I., Kaynig V., Rueden C., Eliceiri K.W., Schindelin J., Cardona A., Sebastian Seung H. Trainable Weka segmentation: a machine learning tool for microscopy pixel classification. Bioinformatics. 2017;33:2424–2426. - PubMed
-
- Ashburner M., Golic K.G., Hawley R.S. Cold Spring Harbor Laboratory Press; 2005. Drosophila: A Laboratory Handbook.
-
- Bargmann C.I., Marder E. From the connectome to brain function. Nat. Methods. 2013;10:483–490. - PubMed
-
- Bay H., Ess A., Tuytelaars T., Van Gool L. Speeded-up robust features (SURF) Comput. Vis. Image Underst. 2008;110:346–359.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

