Cardiac Stem Cells in the Postnatal Heart: Lessons from Development
- PMID: 30034478
- PMCID: PMC6035836
- DOI: 10.1155/2018/1247857
Cardiac Stem Cells in the Postnatal Heart: Lessons from Development
Abstract
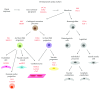
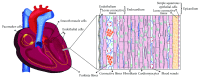
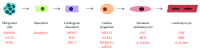
Heart development in mammals is followed by a postnatal decline in cell proliferation and cell renewal from stem cell populations. A better understanding of the developmental changes in cardiac microenvironments occurring during heart maturation will be informative regarding the loss of adult regenerative potential. We reevaluate the adult heart's mitotic potential and the reported adult cardiac stem cell populations, as these are two topics of ongoing debate. The heart's early capacity for cell proliferation driven by progenitors and reciprocal signalling is demonstrated throughout development. The mature heart architecture and environment may be more restrictive on niches that can host progenitor cells. The engraftment issues observed in cardiac stem cell therapy trials using exogenous stem cells may indicate a lack of supporting stem cell niches, while tissue injury adds to a hostile microenvironment for transplanted cells. Engraftment may be improved by preconditioning the cultured stem cells and modulating the microenvironment to host these cells. These prospective areas of further research would benefit from a better understanding of cardiac progenitor interactions with their microenvironment throughout development and may lead to enhanced cardiac niche support for stem cell therapy engraftment.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

