Molecular subtypes and differentiation programmes of glioma stem cells as determinants of extracellular vesicle profiles and endothelial cell-stimulating activities
- PMID: 30034643
- PMCID: PMC6052423
- DOI: 10.1080/20013078.2018.1490144
Molecular subtypes and differentiation programmes of glioma stem cells as determinants of extracellular vesicle profiles and endothelial cell-stimulating activities
Abstract
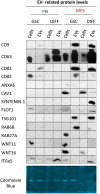
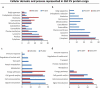
We have previously uncovered the impact of oncogenic and differentiation processes on extracellular vesicles (EVs) in cancer. This is of interested in the context of glioma stem cells (GSC) that are responsible for recurrent nature of glioblastoma multiforme (GBM), while retaining the potential to undergo differentiation and self renewal. GSCs reside in vascular niches where they interact with endothelial cells through a number of mediators including bioactive cargo of EVs. GSCs can be classified as proneural (PN) or mesenchymal (MES) subtypes on the basis of their gene expression profiles and distinct biological characteristics. In the present study we investigated how GSC diversity and differentiation programmes influence their EV-mediated communication potentials. Indeed, molecular subtypes of GBMs and GSCs differ with respect to their expression of EV-related genes (vesiculome) and GSCs with PN or MES phenotypes produce EVs with markedly different characteristics, marker profiles, proteomes and endothelial stimulating activities. For example, while EVs of PN GSC are largely devoid of exosomal markers their counterparts from MES GSCs express ample CD9, CD63 and CD81 tetraspanins. In both GSC subtypes serum-induced differentiation results in profound, but distinct changes of cellular phenotypes including the enhanced EV production, reconfiguration of their proteomes and the related functional pathways. Notably, the EV uptake was a function of both subtype and differentiation state of donor cells. Thus, while, EVs produced by differentiated MES GSCs were internalized less efficiently than those from undifferentiated cells they exhibited an increased stimulatory potential for human brain endothelial cells. Such stimulating activity was also observed for EVs derived from differentiated PN GSCs, despite their even weaker uptake by endothelial cells. These findings suggest that the role of EVs as biological mediators and biomarkers in GBM may depend on the molecular subtype and functional state of donor cancer cells, including cancer stem cells. Abbreviations: CryoTEM: cryo-transmission electron microscopy; DIFF: differentiated GSCs; EGF: epidermal growth factor; DUC: differential ultracentrifugation; EV: extracellular vesicle; FGF: fibroblast growth factor; GBM: glioblastoma multiforme; GFAP: glial fibrillary acidic protein; GO: gene ontology; GSC: glioma stem cells; HBEC-5i: human brain endothelial cells; MES: mesenchymal cells; MTS - [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; PMT1: proneural-to-mesenchyman transition cell line 1; PN: proneural cells; TEM: transmission electron microscopy; WB: western blotting.
Keywords: Glioblastoma; angiogenesis; extracellular vesicles; glioma stem cells; proteome.
Figures







References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

