Rhodium catalyzed diastereoselective synthesis of 2,2,3,3-tetrasubstituted indolines from N-sulfonyl-1,2,3-triazoles and ortho-vinylanilines
- PMID: 30034735
- PMCID: PMC6024588
- DOI: 10.1039/c6sc01075j
Rhodium catalyzed diastereoselective synthesis of 2,2,3,3-tetrasubstituted indolines from N-sulfonyl-1,2,3-triazoles and ortho-vinylanilines
Abstract
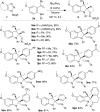
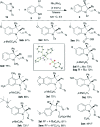
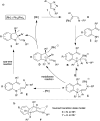
An efficient diastereoselective rhodium catalyzed synthesis of indolines possessing two contiguous tetrasubstituted carbon centers has been achieved with good to excellent yields using ortho-vinylanilines and iminocarbenes derived from N-sulfonyl-1,2,3-triazoles. The reaction affords excellent cis-diastereoselectivity through the initial formation of a N-ylide followed by intramolecular trapping with unactivated alkenes via an ene-type reaction with a well-organized transition state, namely intramolecular carbenylative amination of alkenes. The developed transformation was further extended to the successful synthesis of tricyclic compounds, imidazoindolines, through reduction and hypervalent iodine mediated oxidative cyclization.
Figures
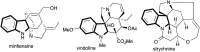



References
-
- Ramirez A., Garcia-Rubio S. Curr. Med. Chem. 2003;10:1891–1915. - PubMed
- Liu J., Zhu J., Tang L., Wen W., Lv S., Yu R. World J. Microbiol. Biotechnol. 2014;30:175–180. - PubMed
- Serasanambati M., Chilakapati S. R., Manikonda P. K., Kanala J. R., Chilakapati D. R. Nat. Prod. Res. 2014;29:484–490. - PubMed
- Chen J., Qu Y., Wang D., Peng P., Cai H., Gao Y., Chen Z., Cai B. Molecules. 2014;19:4395–4408. - PMC - PubMed
-
- Zhang D., Song H., Qin Y. Acc. Chem. Res. 2011;44:447–457. - PubMed
-
- Minatti A., Buchwald S. L. Org. Lett. 2008;10:2721–2724. - PMC - PubMed
- Dounay A. B., Humphreys P. G., Overman L. E., Wrobleski A. D. J. Am. Chem. Soc. 2008;130:5368–5377. - PMC - PubMed
- Yasui Y., Kinugawa T., Takemoto Y. Chem. Commun. 2009:4275. - PubMed
- Watanabe T., Oishi S., Fujii N., Ohno H. Org. Lett. 2008;10:1759–1762. - PubMed
- Schultz D. M., Wolfe J. P. Org. Lett. 2010;12:1028–1031. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

