Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: A review
- PMID: 30034876
- PMCID: PMC6052656
- DOI: 10.1016/j.jare.2018.01.001
Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: A review
Abstract
Our body is endowed with several endogenous anti-microbial compounds such as interferon, cytokines, free radicals, etc. However, little attention has been paid to the possibility that lipids could function as antimicrobial compounds. In this short review, the antimicrobial actions of various polyunsaturated fatty acids (PUFAs, mainly free acids) and their putative mechanisms of action are described. In general, PUFAs kill microbes by their direct action on microbial cell membranes, enhancing generation of free radicals, augmenting the formation of lipid peroxides that are cytotoxic, and by increasing the formation of their bioactive metabolites, such as prostaglandins, lipoxins, resolvins, protectins and maresins that enhance the phagocytic action of leukocytes and macrophages. Higher intakes of α-linolenic and cis-linoleic acids (ALA and LA respectively) and fish (a rich source of eicosapentaenoic acid and docosahexaenoic acid) might reduce the risk pneumonia. Previously, it was suggested that polyunsaturated fatty acids (PUFAs): linoleic, α-linolenic, γ-linolenic (GLA), dihomo-GLA (DGLA), arachidonic (AA), eicosapentaenoic (EPA), and docosahexaenoic acids (DHA) function as endogenous anti-bacterial, anti-fungal, anti-viral, anti-parasitic, and immunomodulating agents. A variety of bacteria are sensitive to the growth inhibitory actions of LA and ALA in vitro. Hydrolyzed linseed oil can kill methicillin-resistant Staphylococcus aureus. Both LA and AA have the ability to inactivate herpes, influenza, Sendai, and Sindbis virus within minutes of contact. AA, EPA, and DHA induce death of Plasmodium falciparum both in vitro and in vivo. Prostaglandin E1 (PGE1) and prostaglandin A (PGA), derived from DGLA, AA, and EPA inhibit viral replication and show anti-viral activity. Oral mucosa, epidermal cells, lymphocytes and macrophages contain and release significant amounts of PUFAs on stimulation. PUFAs stimulate NADPH-dependent superoxide production by macrophages, neutrophils and lymphocytes to kill the invading microorganisms. Cytokines induce the release of PUFAs from cell membrane lipid pool, a potential mechanism for their antimicrobial action. AA, EPA, and DHA give rise to lipoxins (LXs), resolvins, protectins, and maresins that limit and resolve inflammation and have antimicrobial actions. Thus, PUFAs and their metabolites have broad antimicrobial actions.
Keywords: Cytokines; Free radicals; Lipoxin A4; Microbicidal; Prostaglandins; Unsaturated fatty acids.
Figures
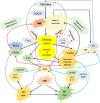
 Black lines indicate normal physiological process.
Black lines indicate normal physiological process.  Red lines indicate inflammatory events or molecules involved in inflammation or inflammation related events such as formation of lipid peroxides that have antimicrobial action.
Red lines indicate inflammatory events or molecules involved in inflammation or inflammation related events such as formation of lipid peroxides that have antimicrobial action.  Green lines indicate anti-inflammatory events or molecules.
Green lines indicate anti-inflammatory events or molecules.  Indicates interaction among ROS and NO, CO and H2S.
Indicates interaction among ROS and NO, CO and H2S.  Blue lines indicate interaction among pro-inflammatory cytokines, ROS and M1 macrophages. PGD2 is known to have both pro- and anti-inflammatory actions (though predominantly anti-inflammatory actions). Since both PGE2 and PGD2 are derived from the precursor PGH2, suggesting that, perhaps, there is a balance maintained between PGE2 and PGD2. PGI2 (not shown in the figure) is also derived from PGH2 that also has anti-inflammatory actions. When microorganisms invade the tissues, they are first encountered by PMNs and macrophages that leads to activation of PLA2 of the cell membrane. Consequently, PUFAs, especially AA/EPA/DHA; are released that are utilized for the formation of PGs, LTs, TXs (that have pro-inflammatory actions) and lipoxins/resolvins/protectins/maresins that have anti-inflammatory actions. In the initial stages, macrophages (M1 type) release IL-6 and TNF-α and PGE2 and LTs to initiate inflammation and eliminate the invading organisms by a mechanism that is dependent on generation of reactive oxygen species (ROS). Once inflammation reaches an optimal level, PGE2/PGD2/PGJ2 activate PLA2 for the release of second wave of AA/EPA/DHA that leads to the formation of anti-inflammatory lipoxins/resolvins/protectins/maresins and convert M1 to M2 macrophages by the release of IL-4, IL-10, IL-12 and IL-13. Macrophages when ingest dead PMNs (efferocytosis) they are triggered to become M2 macrophages due to the release of IL-4/IL-13 and exposure to Axl, C1q and Mertk and formation of lipoxins/resolvins/protectins/maresins that further enhances phagocytosis of M2 macrophages and kills ingested microorganisms and initiates resolution of inflammation and enhances wound healing. The exact initial source of these anti-inflammatory cytokines and bioactive lipids is not clear but may include local tissues involved in inflammation, PMNs and macrophages. It is known that ROS generated by PMNs and macrophages act on AA/EPA/DHA and lead to the formation of respective lipid peroxides that show antimicrobial action. These results also emphasize the close interaction among PMNs, macrophages, T cells, local tissues/cells and invading organisms. This delicate balance between pro- vs anti-inflammatory cytokines and lipids and M1 vs M2 macrophages and ROS vs anti-oxidants is essential to maintain tissue homeostasis and restore physiology to normal. For more details see text.
Blue lines indicate interaction among pro-inflammatory cytokines, ROS and M1 macrophages. PGD2 is known to have both pro- and anti-inflammatory actions (though predominantly anti-inflammatory actions). Since both PGE2 and PGD2 are derived from the precursor PGH2, suggesting that, perhaps, there is a balance maintained between PGE2 and PGD2. PGI2 (not shown in the figure) is also derived from PGH2 that also has anti-inflammatory actions. When microorganisms invade the tissues, they are first encountered by PMNs and macrophages that leads to activation of PLA2 of the cell membrane. Consequently, PUFAs, especially AA/EPA/DHA; are released that are utilized for the formation of PGs, LTs, TXs (that have pro-inflammatory actions) and lipoxins/resolvins/protectins/maresins that have anti-inflammatory actions. In the initial stages, macrophages (M1 type) release IL-6 and TNF-α and PGE2 and LTs to initiate inflammation and eliminate the invading organisms by a mechanism that is dependent on generation of reactive oxygen species (ROS). Once inflammation reaches an optimal level, PGE2/PGD2/PGJ2 activate PLA2 for the release of second wave of AA/EPA/DHA that leads to the formation of anti-inflammatory lipoxins/resolvins/protectins/maresins and convert M1 to M2 macrophages by the release of IL-4, IL-10, IL-12 and IL-13. Macrophages when ingest dead PMNs (efferocytosis) they are triggered to become M2 macrophages due to the release of IL-4/IL-13 and exposure to Axl, C1q and Mertk and formation of lipoxins/resolvins/protectins/maresins that further enhances phagocytosis of M2 macrophages and kills ingested microorganisms and initiates resolution of inflammation and enhances wound healing. The exact initial source of these anti-inflammatory cytokines and bioactive lipids is not clear but may include local tissues involved in inflammation, PMNs and macrophages. It is known that ROS generated by PMNs and macrophages act on AA/EPA/DHA and lead to the formation of respective lipid peroxides that show antimicrobial action. These results also emphasize the close interaction among PMNs, macrophages, T cells, local tissues/cells and invading organisms. This delicate balance between pro- vs anti-inflammatory cytokines and lipids and M1 vs M2 macrophages and ROS vs anti-oxidants is essential to maintain tissue homeostasis and restore physiology to normal. For more details see text.
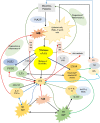
 Black lines indicate normal physiological events.
Black lines indicate normal physiological events.  Red lines indicate pro-inflammatory events/molecules
Red lines indicate pro-inflammatory events/molecules  Indicate molecules involved in immune evasion/immunosuppression AA released from the cell membrane lipid pool by the action of phospholipase A2 (PLA2) can be acted upon by COX-2 to get converted into pro-inflammatory eicosanoids to produce inflammation seen in many microbial infections and also suppress immune response and aid in the growth of tumor cells. AA can induce generation of ROS in immunocytes (leukocytes, macrophages and T and B cells) that, in turn, act on AA to enhance formation of lipid peroxides that are toxic to microbes including viruses and fungi and intracellular parasites. AA can inhibit bacterial Enoyl-acyl carrier protein reductase (Fabl) and thus, produce its bactericidal action. AA can enhance neutral sphingomyelinase activity that enhances ceramide formation, a tumoricidal molecule. It is likely that decreased neutral sphingomyelinase activity drives immune evasion and facilitates tumor growth and thus, PUFAs by virtue of their ability to enhance SMase activity can induce significant enhancement of Th1-mediated and cytotoxic T-cell-mediated antitumor immunity, and by virtue of their ability to enhance synthesis and action of TNF-α and other cytokines and COX-2 expression. In addition, AA can be converted to lipoxin A4, a potent anti-inflammatory and inflammation resolution molecule that can suppress COX-2 activity and inhibit production of pro-inflammatory prostaglandins, thromboxanes and leukotrienes; ROS and alter nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S) generation and thus, aid in the resolution of inflammation and enhance wound healing. Furthermore, LXA4 augments macrophage and PMNs phagocytic activity and thus, scavenge debris at the site of inflammation. It is not yet clear whether LXA4 and lipid peroxides can alter neutral sphingomyelinase activity. It is likely that activated macrophages release AA and corresponding lipid peroxides that, in turn may induce apoptosis of tumor cells. For further details see the text.
Indicate molecules involved in immune evasion/immunosuppression AA released from the cell membrane lipid pool by the action of phospholipase A2 (PLA2) can be acted upon by COX-2 to get converted into pro-inflammatory eicosanoids to produce inflammation seen in many microbial infections and also suppress immune response and aid in the growth of tumor cells. AA can induce generation of ROS in immunocytes (leukocytes, macrophages and T and B cells) that, in turn, act on AA to enhance formation of lipid peroxides that are toxic to microbes including viruses and fungi and intracellular parasites. AA can inhibit bacterial Enoyl-acyl carrier protein reductase (Fabl) and thus, produce its bactericidal action. AA can enhance neutral sphingomyelinase activity that enhances ceramide formation, a tumoricidal molecule. It is likely that decreased neutral sphingomyelinase activity drives immune evasion and facilitates tumor growth and thus, PUFAs by virtue of their ability to enhance SMase activity can induce significant enhancement of Th1-mediated and cytotoxic T-cell-mediated antitumor immunity, and by virtue of their ability to enhance synthesis and action of TNF-α and other cytokines and COX-2 expression. In addition, AA can be converted to lipoxin A4, a potent anti-inflammatory and inflammation resolution molecule that can suppress COX-2 activity and inhibit production of pro-inflammatory prostaglandins, thromboxanes and leukotrienes; ROS and alter nitric oxide (NO), carbon monoxide (CO) and hydrogen sulfide (H2S) generation and thus, aid in the resolution of inflammation and enhance wound healing. Furthermore, LXA4 augments macrophage and PMNs phagocytic activity and thus, scavenge debris at the site of inflammation. It is not yet clear whether LXA4 and lipid peroxides can alter neutral sphingomyelinase activity. It is likely that activated macrophages release AA and corresponding lipid peroxides that, in turn may induce apoptosis of tumor cells. For further details see the text.Similar articles
-
"Cell Membrane Theory of Senescence" and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications.Biomolecules. 2021 Feb 8;11(2):241. doi: 10.3390/biom11020241. Biomolecules. 2021. PMID: 33567774 Free PMC article. Review.
-
Cytokines, angiogenic, and antiangiogenic factors and bioactive lipids in preeclampsia.Nutrition. 2015 Sep;31(9):1083-95. doi: 10.1016/j.nut.2015.03.013. Epub 2015 Apr 29. Nutrition. 2015. PMID: 26233865 Review.
-
Effect of polyunsaturated fatty acids and their metabolites on bleomycin-induced cytotoxic action on human neuroblastoma cells in vitro.PLoS One. 2014 Dec 23;9(12):e114766. doi: 10.1371/journal.pone.0114766. eCollection 2014. PLoS One. 2014. PMID: 25536345 Free PMC article.
-
COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance.Eur J Pharmacol. 2016 Aug 15;785:116-132. doi: 10.1016/j.ejphar.2015.08.049. Epub 2015 Sep 1. Eur J Pharmacol. 2016. PMID: 26335394 Review.
-
Can Bioactive Lipids Inactivate Coronavirus (COVID-19)?Arch Med Res. 2020 Apr;51(3):282-286. doi: 10.1016/j.arcmed.2020.03.004. Epub 2020 Mar 27. Arch Med Res. 2020. PMID: 32229155 Free PMC article.
Cited by
-
Integrated metabolomics, network pharmacology and biological verification to reveal the mechanisms of Nauclea officinalis treatment of LPS-induced acute lung injury.Chin Med. 2022 Nov 24;17(1):131. doi: 10.1186/s13020-022-00685-6. Chin Med. 2022. PMID: 36434729 Free PMC article.
-
Gut germinal center regeneration and enhanced antiviral immunity by mesenchymal stem/stromal cells in SIV infection.JCI Insight. 2021 Jun 22;6(12):e149033. doi: 10.1172/jci.insight.149033. JCI Insight. 2021. PMID: 34014838 Free PMC article.
-
Application of Acidulants to Control Salmonella spp. in Rendered Animal Fats and Oils with Different Levels of Unsaturation.Animals (Basel). 2023 Apr 11;13(8):1304. doi: 10.3390/ani13081304. Animals (Basel). 2023. PMID: 37106867 Free PMC article.
-
Extensive remodelling of the cell wall during the development of Staphylococcus aureus bacteraemia.Elife. 2023 Jul 4;12:RP87026. doi: 10.7554/eLife.87026. Elife. 2023. PMID: 37401629 Free PMC article.
-
Fungal Quorum-Sensing Molecules and Inhibitors with Potential Antifungal Activity: A Review.Molecules. 2019 May 21;24(10):1950. doi: 10.3390/molecules24101950. Molecules. 2019. PMID: 31117232 Free PMC article. Review.
References
-
- Speert D.P., Quie P.G., Wannamaker L.W. Enhanced phagocytosis of group A Streptococci M type 6 by oleic acid. J Infect Dis. 1981;143:570–577. - PubMed
-
- Lokesh B.R., Wrann M. Incorporation of palmitic acid or oleic acid into macrophage membrane lipids exerts differential effects on the function of normal mouse peritoneal macrophages. Biochim Biophys Acta. 1984;792:141–148. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

