A tailor-made, self-sufficient and recyclable monooxygenase catalyst based on coimmobilized cytochrome P450 BM3 and glucose dehydrogenase
- PMID: 30036448
- PMCID: PMC6836874
- DOI: 10.1002/bit.26802
A tailor-made, self-sufficient and recyclable monooxygenase catalyst based on coimmobilized cytochrome P450 BM3 and glucose dehydrogenase
Abstract
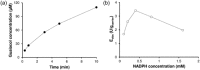
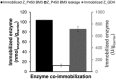
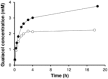
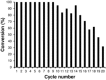
Cytochrome P450 monooxygenases (P450s) promote hydroxylations in a broad variety of substrates. Their prowess in C-H bond functionalization renders P450s promising catalysts for organic synthesis. However, operating P450 reactions involve complex management of the main substrates, O2 and nicotinamide adenine dinucleotide phosphate (NAD(P)H) reducing equivalents against an overall background of low operational stability. Whole-cell biocatalysis, although often used, offers no general solution to these problems. Herein, we present the design of a tailor-made, self-sufficient, operationally stabilized and recyclable P450 catalyst on porous solid support. Using enzymes as fusion proteins with the polycationic binding module Zbasic2 , the P450s BM3 (from Bacillus megaterium) was coimmobilized with glucose dehydrogenase (type IV; from B. megaterium) on anionic sulfopropyl-activated carrier (ReliSorb SP). Immobilization via Zbasic2 enabled each enzyme to be loaded in controllable amount, thus maximizing the relative portion of the rate limiting P450 BM3 (up to 19.5 U/gcarrier ) in total enzyme immobilized. Using lauric acid as a representative P450 substrate that is poorly accessible to whole-cell catalysts, we demonstrate complete hydroxylation at low catalyst loading (≤0.1 mol%) and efficient electron coupling (74%), inside of the catalyst particle, to the regeneration of NADPH from glucose (27 cycles) was achieved. The immobilized P450 BM3 showed a total turnover number of ∼18,000, thus allowing active catalyst to be recycled up to 20 times. This study therefore supports the idea of practical heterogeneous catalysis by P450s systems immobilized on solid support.
Keywords: cytochrome P450; fatty acids; glucose dehydrogenase; heterogeneous biocatalysis; immobilization.
© 2018 Wiley Periodicals, Inc.
Figures



References
-
- Bolivar, J. M. , & Nidetzky, B. (2012). Oriented and selective enzyme immobilization on functionalized silica carrier using the cationic binding module Zbasic2: Design of a heterogeneous d‐amino acid oxidase catalyst on porous glass. Biotechnology and Bioengineering, 109(6), 1490–1498. 10.1002/bit.24423 - DOI - PubMed
-
- CalcTool. (2018). Retrieved from http://www.calctool.org/CALC/prof/bio/protein_size
-
- Dennig, A. , Busto, E. , Kroutil, W. , & Faber, K. (2015). Biocatalytic one‐pot synthesis of l‐tyrosine derivatives from monosubstituted benzenes, pyruvate, and ammonia. ACS Catalysis, 5(12), 7503–7506. 10.1021/acscatal.5b02129 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

