Low diversity gut microbiota dysbiosis: drivers, functional implications and recovery
- PMID: 30036705
- PMCID: PMC6435260
- DOI: 10.1016/j.mib.2018.07.003
Low diversity gut microbiota dysbiosis: drivers, functional implications and recovery
Abstract
Dysbiosis, an imbalance in microbial communities, is linked with disease when this imbalance disturbs microbiota functions essential for maintaining health or introduces processes that promote disease. Dysbiosis in disease is predicted when microbiota differ compositionally from a healthy control population, but only truly defined when these differences are mechanistically related to adverse phenotypes. For the human gut microbiota, dysbiosis varies across diseases. One common manifestation is replacement of the complex community of anaerobes typical of the healthy adult gut microbiome with a community of lower overall microbial diversity and increased facultative anaerobes. Here we review diseases in which low-diversity dysbiosis has been observed and mechanistically linked with disease, with a particular focus on liver disease, inflammatory bowel disease, and Clostridium difficile infection.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
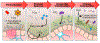
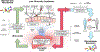
References
-
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI: Host-bacterial mutualism in the human intestine. Science 2005, 307:1915–1920. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

