Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives
- PMID: 30036727
- PMCID: PMC6710094
- DOI: 10.1016/j.biomaterials.2018.07.017
Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives
Abstract
Bone fractures are the most common traumatic injuries in humans. The repair of bone fractures is a regenerative process that recapitulates many of the biological events of embryonic skeletal development. Most of the time it leads to successful healing and the recovery of the damaged bone. Unfortunately, about 5-10% of fractures will lead to delayed healing or non-union, more so in the case of co-morbidities such as diabetes. In this article, we review the different strategies to heal bone defects using synthetic bone graft substitutes, biologically active substances and stem cells. The majority of currently available reviews focus on strategies that are still at the early stages of development and use mostly in vitro experiments with cell lines or stem cells. Here, we focus on what is already implemented in the clinics, what is currently in clinical trials, and what has been tested in animal models. Treatment approaches can be classified in three major categories: i) synthetic bone graft substitutes (BGS) whose architecture and surface can be optimized; ii) BGS combined with bioactive molecules such as growth factors, peptides or small molecules targeting bone precursor cells, bone formation and metabolism; iii) cell-based strategies with progenitor cells combined or not with active molecules that can be injected or seeded on BGS for improved delivery. We review the major types of adult stromal cells (bone marrow, adipose and periosteum derived) that have been used and compare their properties. Finally, we discuss the remaining challenges that need to be addressed to significantly improve the healing of bone defects.
Keywords: Bioactive; Biomaterial; Fracture; Nonunion; Scaffold; Stem cells.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
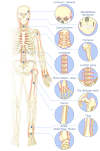

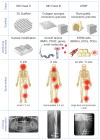

References
-
- Inc O. In: The orthopaedic industry annual report : 2010-2011. Inc O, editor. Ohio, Chagrin Falls: 44 0232010-2011.
-
- Holmes D. Non-union bone fracture: a quicker fix. Nature. 2017;550:S193. - PubMed
-
- Holmes D. Closing the gap. Nature. 2017;550:S194–S5. - PubMed
-
- Seeman E. Bone quality: the material and structural basis of bone strength. J Bone Miner Metab. 2008;26:1–8. - PubMed
-
- Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

