The Keap1/Nrf2-ARE Pathway as a Pharmacological Target for Chalcones
- PMID: 30037040
- PMCID: PMC6100069
- DOI: 10.3390/molecules23071803
The Keap1/Nrf2-ARE Pathway as a Pharmacological Target for Chalcones
Abstract
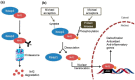
Chalcones have shown a broad spectrum of biological activities with clinical potential against various diseases. The biological activities are mainly attributed to the presence in the chalcones of the α,β-unsaturated carbonyl system, perceived as a potential Michael acceptor. Chalcones could activate the Kelch-like ECH-associated protein 1 (Keap1)/Nuclear factor erythroid 2-related factor 2 (Nrf2) pathway through a Michael addition reaction with the cysteines of Keap1, which acts as a redox sensor and negative regulator of Nrf2. This modification allows the dissociation of Nrf2 from the cytoplasmic complex with Keap1 and its nuclear translocation. At this level, Nrf2 binds to the antioxidant response element (ARE) and activates the expression of several detoxification, antioxidant and anti-inflammatory genes as well as genes involved in the clearance of damaged proteins. In this regard, the Keap1/Nrf2⁻ARE pathway is a new potential pharmacological target for the treatment of many chronic diseases. In this review we summarize the current progress in the study of Keap1/Nrf2⁻ARE pathway activation by natural and synthetic chalcones and their potential pharmacological applications. Among the pharmacological activities highlighted, anti-inflammatory activity was more evident than others, suggesting a multi-target Michael acceptor mechanism for the chalcones involving key regulators of the Nrf2 and nuclear factor- κB (NF-κB) pathways.
Keywords: Keap1; NF-κB; Nrf2; anti-inflammatory activity; antioxidant activity; chalcones; multi-target activity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

