Antibiotic Targets in Gonococcal Cell Wall Metabolism
- PMID: 30037076
- PMCID: PMC6164560
- DOI: 10.3390/antibiotics7030064
Antibiotic Targets in Gonococcal Cell Wall Metabolism
Abstract
The peptidoglycan cell wall that encloses the bacterial cell and provides structural support and protection is remodeled by multiple enzymes that synthesize and cleave the polymer during growth. This essential and dynamic structure has been targeted by multiple antibiotics to treat gonococcal infections. Up until now, antibiotics have been used against the biosynthetic machinery and the therapeutic potential of inhibiting enzymatic activities involved in peptidoglycan breakdown has not been explored. Given the major antibiotic resistance problems we currently face, it is crucial to identify other possible targets that are key to maintaining cell integrity and contribute to disease development. This article reviews peptidoglycan as an antibiotic target, how N. gonorrhoeae has developed resistance to currently available antibiotics, and the potential of continuing to target this essential structure to combat gonococcal infections by attacking alternative enzymatic activities involved in cell wall modification and metabolism.
Keywords: Neisseria gonorrhoeae; lytic transglycosylase; peptidoglycan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
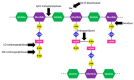
References
-
- Centers for Disease Control Antibiotic Resistance Threats in the United States. [(accessed on 16 April 2018)];2013 Available online: https://www.cdc.gov/drugresistance/threat-report-2013/index.html.
-
- Centers for Disease Control 2015 Sexually Transmitted Diseases Surveillance. [(accessed on 16 April 2018)]; Available online: https://www.cdc.gov/std/stats15/gonorrhea.htm.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

