Continuous low-dose antibiotic prophylaxis for adults with repeated urinary tract infections (AnTIC): a randomised, open-label trial
- PMID: 30037647
- PMCID: PMC6105581
- DOI: 10.1016/S1473-3099(18)30279-2
Continuous low-dose antibiotic prophylaxis for adults with repeated urinary tract infections (AnTIC): a randomised, open-label trial
Erratum in
-
Corrections.Lancet Infect Dis. 2018 Sep;18(9):941. doi: 10.1016/S1473-3099(18)30504-8. Lancet Infect Dis. 2018. PMID: 30152351 Free PMC article. No abstract available.
Abstract
Background: Repeated symptomatic urinary tract infections (UTIs) affect 25% of people who use clean intermittent self-catheterisation (CISC) to empty their bladder. We aimed to determine the benefits, harms, and cost-effectiveness of continuous low-dose antibiotic prophylaxis for prevention of recurrent UTIs in adult users of CISC.
Methods: In this randomised, open-label, superiority trial, we enrolled participants from 51 UK National Health Service organisations. These participants were community-dwelling (as opposed to hospital inpatient) users of CISC with recurrent UTIs. We randomly allocated participants (1:1) to receive either antibiotic prophylaxis once daily (prophylaxis group) or no prophylaxis (control group) for 12 months by use of an internet-based system with permuted blocks of variable length. Trial and laboratory staff who assessed outcomes were masked to allocation but participants were aware of their treatment group. The primary outcome was the incidence of symptomatic, antibiotic-treated UTIs over 12 months. Participants who completed at least 6 months of follow-up were assumed to provide a reliable estimate of UTI incidence and were included in the analysis of the primary outcome. Change in antimicrobial resistance of urinary and faecal bacteria was monitored as a secondary outcome. The AnTIC trial is registered at ISRCTN, number 67145101; and EudraCT, number 2013-002556-32.
Findings: Between Nov 25, 2013, and Jan 29, 2016, we screened 1743 adult users of CISC for eligibility, of whom 404 (23%) participants were enrolled between Nov 26, 2013, and Jan 31, 2016. Of these 404 participants, 203 (50%) were allocated to receive prophylaxis and 201 (50%) to receive no prophylaxis. 1339 participants were excluded before randomisation. The primary analysis included 181 (89%) adults allocated to the prophylaxis group and 180 (90%) adults in the no prophylaxis (control) group. 22 participants in the prophylaxis group and 21 participants in the control group were not included in the primary analysis because they were missing follow-up data before 6 months. The incidence of symptomatic antibiotic-treated UTIs over 12 months was 1·3 cases per person-year (95% CI 1·1-1·6) in the prophylaxis group and 2·6 (2·3-2·9) in the control group, giving an incidence rate ratio of 0·52 (0·44-0·61; p<0·0001), indicating a 48% reduction in UTI frequency after treatment with prophylaxis. Use of prophylaxis was well tolerated: we recorded 22 minor adverse events in the prophylaxis group related to antibiotic prophylaxis during the study, predominantly gastrointestinal disturbance (six participants), skin rash (six participants), and candidal infection (four participants). However, resistance against the antibiotics used for UTI treatment was more frequent in urinary isolates from the prophylaxis group than in those from the control group at 9-12 months of trial participation (nitrofurantoin 12 [24%] of 51 participants from the prophylaxis group vs six [9%] of 64 participants from the control group with at least one isolate; p=0·038), trimethoprim (34 [67%] of 51 vs 21 [33%] of 64; p=0·0003), and co-trimoxazole (26 [53%] of 49 vs 15 [24%] of 62; p=0·002).
Interpretation: Continuous antibiotic prophylaxis is effective in reducing UTI frequency in CISC users with recurrent UTIs, and it is well tolerated in these individuals. However, increased resistance of urinary bacteria is a concern that requires surveillance if prophylaxis is started.
Funding: UK National Institute for Health Research.
Copyright © 2018 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
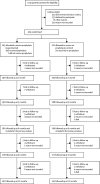
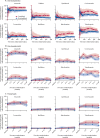
Comment in
-
Preventing urinary tract infections in patients with neurogenic bladder.Lancet Infect Dis. 2018 Sep;18(9):926-927. doi: 10.1016/S1473-3099(18)30284-6. Epub 2018 Jun 28. Lancet Infect Dis. 2018. PMID: 30153930 No abstract available.
-
Antibiotic prophylaxis approaches for urinary tract infections.Lancet Infect Dis. 2018 Oct;18(10):1065. doi: 10.1016/S1473-3099(18)30501-2. Lancet Infect Dis. 2018. PMID: 30303098 No abstract available.
-
Re: Continuous Low-Dose Antibiotic Prophylaxis for Adults with Repeated Urinary Tract Infections (AnTIC): A Randomised, Open-Label Trial.J Urol. 2019 Aug;202(2):191. doi: 10.1097/JU.0000000000000302. Epub 2019 Jul 8. J Urol. 2019. PMID: 31021292 No abstract available.
-
Re: Continuous Low-dose Antibiotic Prophylaxis for Adults with Repeated Urinary Tract Infections (AnTIC): A Randomized, Open-label Trial.Eur Urol. 2019 Nov;76(5):708. doi: 10.1016/j.eururo.2019.05.018. Epub 2019 May 28. Eur Urol. 2019. PMID: 31151675 No abstract available.
References
-
- Wyndaele JJ. Complications of intermittent catheterization: their prevention and treatment. Spinal Cord. 2002;40:536–541. - PubMed
-
- Bakke A. Clean intermittent catheterization—physical and psychological complications. Scand J Urol Nephrol Suppl. 1993;150:1–69. - PubMed
-
- Niël-Weise BS, van den Broek PJ, da Silva EM, Silva LA. Urinary catheter policies for long-term bladder drainage. Cochrane Database Syst Rev. 2012;8 CD004201. - PubMed
-
- Goff DA, Mendelson M. Antibiotic stewardship hits a home run for patients. Lancet Infect Dis. 2017;17:892–893. - PubMed
-
- Gribble MJ, Puterman ML. Prophylaxis of urinary tract infection in persons with recent spinal cord injury: a prospective, randomized, double-blind, placebo-controlled study of trimethoprim-sulfamethoxazole. Am J Med. 1993;95:141–152. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

