Donepezil Treatment in Patients With Depression and Cognitive Impairment on Stable Antidepressant Treatment: A Randomized Controlled Trial
- PMID: 30037778
- PMCID: PMC6396676
- DOI: 10.1016/j.jagp.2018.05.008
Donepezil Treatment in Patients With Depression and Cognitive Impairment on Stable Antidepressant Treatment: A Randomized Controlled Trial
Abstract
Objective: Depression and cognitive impairment are often comorbid in older adults, but optimal treatment strategies remain unclear. In a two-site study, the efficacy and safety of add-on donepezil versus placebo were compared in depressed patients with cognitive impairment receiving stable antidepressant treatment.
Methods: A randomized, double-blind, placebo-controlled trial was conducted in older adults with depression and cognitive impairment (https://clinicaltrials.gov/ct2/show/NCT01658228; NCT01658228). Patients received open-label antidepressant treatment for 16 weeks, initially with citalopram and then with venlafaxine, if needed, followed by random assignment to add-on donepezil 5-10 mg daily or placebo for another 62 weeks. Outcome measures were neuropsychological test performance (Alzheimer's Disease Assessment Scale-Cognitive subscale [ADAS-Cog] and Selective Reminding Test [SRT] total immediate recall) and instrumental activities of daily living (Functional Activities Questionnaire).
Results: Of 81 patients who signed informed consent, 79 patients completed the baseline evaluation. Open antidepressant treatment was associated with improvement in depression in 63.93% responders by week 16. In the randomized trial, there were no treatment group differences between donepezil and placebo on dementia conversion rates, ADAS-Cog, SRT total immediate recall, or FAQ. Neither baseline cognitive impairment severity nor apolipoprotein E e4 genotype influenced donepezil efficacy. Donepezil was associated with more adverse effects than placebo.
Conclusion: The results do not support adjunctive off-label cholinesterase inhibitor treatment in patients with depression and cognitive impairment. The findings highlight the need to prioritize discovery of novel treatments for this highly prevalent population with comorbid illnesses.
Keywords: Mild cognitive impairment; antidepressants; cholinesterase inhibitor; clinical trial; depression.
Copyright © 2018 American Association for Geriatric Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures

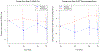
References
-
- Hanninen T, Hallikainen M, Koivisto K, et al.: A follow-up study of age-associated memory impairment: neuropsychological predictors of dementia. J Am Geriatr Soc 1995; 43:1007–1015 - PubMed
-
- Jonker C, Geerlings MI, Schmand B: Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry 2000; 15:983–991 - PubMed
-
- Blazer D, Hughes DC, George LK: The epidemiology of depression in an elderly community population. Gerontologist 1987; 27:281–287 - PubMed
-
- Burt DB, Zembar MJ, Niederehe G: Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull 1995; 117:285–305 - PubMed
-
- Arve S, Tilvis RS, Lehtonen A, et al.: Coexistence of lowered mood and cognitive impairment of elderly people in five birth cohorts. Aging (Milano) 1999; 11:90–95 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

