A Closely Associated Phospholipase C Regulates Cation Channel Function through Phosphoinositide Hydrolysis
- PMID: 30037836
- PMCID: PMC6705966
- DOI: 10.1523/JNEUROSCI.0586-18.2018
A Closely Associated Phospholipase C Regulates Cation Channel Function through Phosphoinositide Hydrolysis
Abstract
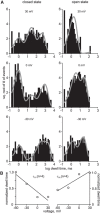
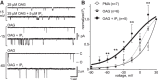
In the hemaphroditic sea snail, Aplysia californica, reproduction is initiated when the bag cell neurons secrete egg-laying hormone during a protracted afterdischarge. A source of depolarization for the afterdischarge is a voltage-gated, nonselective cation channel, similar to transient receptor potential (TRP) channels. Once the afterdischarge is triggered, phospholipase C (PLC) is activated to hydrolyze phosphatidylinositol-4,5-bisphosphate (PIP2) into diacylglycerol (DAG) and inositol trisphosphate (IP3). We previously reported that a DAG analog, 1-oleoyl-2-acetyl-sn-glycerol (OAG), activates a prominent, inward whole-cell cationic current that is enhanced by IP3 To examine the underlying mechanism, we investigated the effect of exogenous OAG and IP3, as well as PLC activation, on cation channel activity and voltage dependence in excised, inside-out patches from cultured bag cell neurons. OAG transiently elevated channel open probability (PO) when applied to excised patches; however, coapplication of IP3 prolonged the OAG-induced response. In patches exposed to OAG and IP3, channel voltage dependence was left-shifted; this was also observed with OAG, but not to the same extent. Introducing the PLC activator, m-3M3FBS, to patches increased channel PO, suggesting PLC may be physically linked to the channels. Accordingly, blocking PLC with U-73122 ablated the m-3M3FBS-induced elevation in PO Treatment with m-3M3FBS left-shifted cation channel voltage dependence to a greater extent than exogenous OAG and IP3 Finally, OAG and IP3 potentiated the stimulatory effect of PKC, which is also associated with the channel. Thus, the PLC-PKC signaling system is physically localized such that PIP2 breakdown products liberated during the afterdischarge modulate the cation channel and temporally influence neuronal activity.SIGNIFICANCE STATEMENT Using excised patches from Aplysia bag cell neurons, we present the first evidence of a nonselective cation channel physically associating with phospholipase C (PLC) at the single-channel level. PLC-mediated breakdown of phospholipids generates diacylglycerol and inositol trisphosphate, which activate the cation channel. This is mimicked by exogenous lipids; furthermore, these second messengers left-shift channel voltage dependence and enhance the response of the channel to protein kinase C. PLC-mediated lipid signaling controls single-channel currents to ensure depolarization is maintained for an extended period of firing, termed the afterdischarge, when the bag cell neurons secrete egg-laying hormone to trigger reproduction.
Keywords: afterdischarge; diacylglycerol; inositol trisphosphate; mollusk; neuroendocrine cell; voltage dependence.
Copyright © 2018 the authors 0270-6474/18/387622-13$15.00/0.
Figures






References
-
- Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S (1990) Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther 255:756–768. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
