Hepatitis A Virus Capsid Structure
- PMID: 30037986
- PMCID: PMC6496327
- DOI: 10.1101/cshperspect.a031807
Hepatitis A Virus Capsid Structure
Abstract
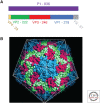
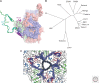
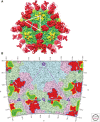
Hepatitis A virus (HAV) has been enigmatic, evading detailed structural analysis for many years. Its recently determined high-resolution structure revealed an angular surface without the indentations often characteristic of receptor-binding sites. The viral protein 1 (VP1) β-barrel shows no sign of a pocket factor and the amino terminus of VP2 displays a "domain swap" across the pentamer interface, as in a subset of mammalian picornaviruses and insect picorna-like viruses. Structure-based phylogeny confirms this placement. These differences suggest an uncoating mechanism distinct from that of enteroviruses. An empty capsid structure reveals internal differences in VP0 and the VP1 amino terminus connected with particle maturation. An HAV/Fab complex structure, in which the antigen-binding fragment (Fab) appears to act as a receptor-mimic, clarifies some historical epitope mapping data, but some remain difficult to reconcile. We still have little idea of the structural features of enveloped HAV particles.
Copyright © 2019 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Acharya R, Fry E, Stuart D, Fox G, Rowlands D, Brown F. 1989. The three-dimensional structure of foot-and-mouth disease virus at 2.9 Å resolution. Nature 337: 709–716. - PubMed
-
- Chapman MS, Rossmann MG. 1993. Comparison of surface properties of picornaviruses: Strategies for hiding the receptor site from immune surveillance. Virology 195: 745–756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
