Population Pharmacokinetics of the Antimalarial Amodiaquine: a Pooled Analysis To Optimize Dosing
- PMID: 30038039
- PMCID: PMC6153844
- DOI: 10.1128/AAC.02193-17
Population Pharmacokinetics of the Antimalarial Amodiaquine: a Pooled Analysis To Optimize Dosing
Abstract
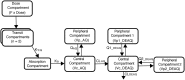
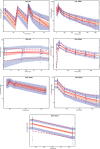
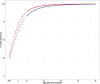
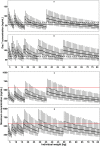
Amodiaquine plus artesunate is the recommended antimalarial treatment in many countries where malaria is endemic. However, pediatric doses are largely based on a linear extrapolation from adult doses. We pooled data from previously published studies on the pharmacokinetics of amodiaquine, to optimize the dose across all age groups. Adults and children with uncomplicated malaria received daily weight-based doses of amodiaquine or artesunate-amodiaquine over 3 days. Plasma concentration-time profiles for both the parent drug and the metabolite were characterized using nonlinear mixed-effects modeling. Amodiaquine pharmacokinetics were adequately described by a two-compartment disposition model, with first-order elimination leading to the formation of desethylamodiaquine, which was best described by a three-compartment disposition model. Body size and age were the main covariates affecting amodiaquine clearance. After adjusting for the effect of weight, clearance rates for amodiaquine and desethylamodiaquine reached 50% of adult maturation at 2.8 months (95% confidence interval [CI], 1.5 to 3.7 months) and 3.9 months (95% CI, 2.6 to 5.3 months) after birth, assuming that the baby was born at term. Bioavailability was 22.4% (95% CI, 15.6 to 31.9%) lower at the start of treatment than during convalescence, which suggests a malaria disease effect. Neither the drug formulation nor the hemoglobin concentration had an effect on any pharmacokinetic parameters. Results from simulations showed that current manufacturer dosing recommendations resulted in low desethylamodiaquine exposure in patients weighing 8 kg, 15 to 17 kg, 33 to 35 kg, and >62 kg compared to that in a typical 50-kg patient. We propose possible optimized dosing regimens to achieve similar drug exposures among all age groups, which require further validation.
Keywords: NONMEM; dose optimization; malaria; pediatrics.
Copyright © 2018 Ali et al.
Figures
References
-
- World Health Organization. 2016. World malaria report. World Health Organization, Geneva, Switzerland.
-
- World Health Organization. 2015. Status report on artemisinin and ACT resistance, p 1–8. World Health Organization, Geneva, Switzerland.
-
- World Health Organization. 2015. World malaria report 2015. World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

