Endocervical and vaginal microbiota in South African adolescents with asymptomatic Chlamydia trachomatis infection
- PMID: 30038262
- PMCID: PMC6056523
- DOI: 10.1038/s41598-018-29320-x
Endocervical and vaginal microbiota in South African adolescents with asymptomatic Chlamydia trachomatis infection
Abstract
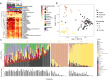
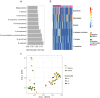
Adolescent girls and young women represent a key risk group for sexually transmitted infections (STIs). The vaginal microbiota is thought to play an important role in susceptibility to STIs such as Chlamydia trachomatis. We compared the microbiota of the lateral vaginal wall and endocervix, and assessed associations with C. trachomatis infection in South African adolescents. The endocervical and vaginal lateral wall microbiota were characterized by amplifying and sequencing the V4 region of the 16S rRNA gene and C. trachomatis diagnosed using molecular methods. Of the 72 girls included, 30 had asymptomatic C. trachomatis infections. Three major vaginal community types were identified; one Lactobacillus crispatus, one L. iners and one diverse, Gardnerella vaginalis dominant. The microbiota of the endocervix was significantly different from that of the lateral wall in terms of diversity. There were many differentially abundant taxa between the endocervix and lateral vaginal wall, including Achromobacter spanius and Enterococcus faecium. Women with C. trachomatis had higher relative abundance of G. vaginalis and other anaerobes. In this African adolescent cohort, significant differences between the lateral vaginal wall and endocervical microbiota diversity and composition were evident, although neither were strongly associated with C. trachomatis infection.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

