Human Mast Cell Tryptase Is a Potential Treatment for Snakebite Envenoming Across Multiple Snake Species
- PMID: 30038613
- PMCID: PMC6047305
- DOI: 10.3389/fimmu.2018.01532
Human Mast Cell Tryptase Is a Potential Treatment for Snakebite Envenoming Across Multiple Snake Species
Abstract
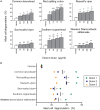
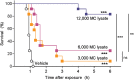
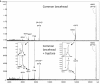
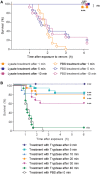
Snake envenoming is a serious and neglected public health crisis that is responsible for as many as 125,000 deaths per year, which is one of the reasons the World Health Organization has recently reinstated snakebite envenoming to its list of category A neglected tropical diseases. Here, we investigated the ability of human mast cell proteases to detoxify six venoms from a spectrum of phylogenetically distinct snakes. To this end, we developed a zebrafish model to assess effects on the toxicity of the venoms and characterized the degradation of venom proteins by mass spectrometry. All snake venoms tested were detoxified by degradation of various venom proteins by the mast cell protease tryptase β, and not by other proteases. Our data show that recombinant human tryptase β degrades and detoxifies a phylogenetically wide range of venoms, indicating that recombinant human tryptase could possibly be developed as a universal antidote to venomous snakebites.
Keywords: antivenom; mast cell; proteases; snakes; venom.
Figures







References
LinkOut - more resources
Full Text Sources
Other Literature Sources

