CDK4/6 inhibition in breast cancer: current practice and future directions
- PMID: 30038670
- PMCID: PMC6050811
- DOI: 10.1177/1758835918786451
CDK4/6 inhibition in breast cancer: current practice and future directions
Abstract
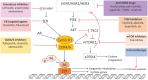
The cyclin D/cyclin-dependent kinases 4 and 6 (CDK4/6)-retinoblastoma protein (RB) pathway plays a key role in the proliferation of both normal breast epithelium and breast cancer cells. A strong rationale for inhibiting CDK4/6 in breast cancers has been present for many years. However, potent and selective CDK4/6 inhibitors have only recently become available. These agents prevent phosphorylation of the RB tumor suppressor, thereby invoking cancer cell cycle arrest in G1. CDK4/6 inhibitors have transited rapidly from preclinical studies to the clinical arena, and three have already been approved for the treatment of advanced, estrogen receptor (ER)-positive breast cancer patients on account of striking clinical trial results demonstrating substantial improvements in progression-free survival. ER-positive breast cancers harbor several molecular features that would predict their sensitivity to CDK4/6 inhibitors. As physicians gain experience with using these agents in the clinic, new questions arise: are CDK4/6 inhibitors likely to be useful for patients with other subtypes of breast cancer? Are there other agents that could be effectively combined with CDK4/6 inhibitors, beyond endocrine therapy? Is there a rationale for combining CDK4/6 inhibitors with novel immune-based therapies? In this review, we describe not only the clinical data available to date, but also the biology of the CDK4/6 pathway and discuss answers to these questions. In particular, we highlight that CDK4 and CDK6 govern much more than the cancer cell cycle, and that their optimal use in the clinic depends on a deeper understanding of the less well characterized effects of these enzymes.
Keywords: CDK4/6 inhibitors; HER2-positive; PI3K inhibitors; breast cancer; immune checkpoint blockade; predictors of response/resistance; triple-negative breast cancer.
Conflict of interest statement
Conflict of interest statement: S. Pernas has received honoraria for talks and travel grants from Roche, outside of the submitted work, and has served on advisory boards for Polyphor. S. Goel performs laboratory research sponsored by Eli Lilly and serves as a paid adviser to Eli Lilly. S. Tolaney receives institutional research funding from Eli Lilly, Pfizer, Novartis, Exelixis, Eisai, Merck, Bristol Meyers Squibb, AstraZeneca, Nektar. S. Tolaney has also served on advisory boards for Eli Lilly, Pfizer, Novartis, Eisai, Merck, AstraZeneca, Nektar, and Puma. E Winer has been a consultant to Roche and Eli Lilly.
Figures
Comment in
-
Ribociclib-induced hypocalcemia in metastatic breast cancer.Indian J Cancer. 2022 Jan-Mar;59(1):140-141. doi: 10.4103/ijc.IJC_1027_20. Indian J Cancer. 2022. PMID: 35645058 No abstract available.
References
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. - PubMed
-
- Arnold A, Papanikolaou A. Cyclin D1 in breast cancer pathogenesis. J Clin Oncol 2005; 23: 4215–4224. - PubMed
-
- Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature 2001; 411: 1017–1021. - PubMed
-
- Yu Q, Sicinska E, Geng Y, et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell 2006; 9: 23–32. - PubMed
-
- Sharpless NE, Sherr CJ. Forging a signature of in vivo senescence. Nat Rev Cancer 2015; 15: 397–408. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

