Frequency of Loss of Function Variants in LRRK2 in Parkinson Disease
- PMID: 30039155
- PMCID: PMC6248108
- DOI: 10.1001/jamaneurol.2018.1885
Frequency of Loss of Function Variants in LRRK2 in Parkinson Disease
Abstract
Importance: Pathogenic variants in LRRK2 are a relatively common genetic cause of Parkinson disease (PD). Currently, the molecular mechanism underlying disease is unknown, and gain and loss of function (LOF) models of pathogenesis have been postulated. LRRK2 variants are reported to result in enhanced phosphorylation of substrates and increased cell death. However, the double knockout of Lrrk2 and its homologue Lrrk1 results in neurodegeneration in a mouse model, suggesting that disease may occur by LOF. Because LRRK2 inhibitors are currently in development as potential disease-modifying treatments in PD, it is critical to determine whether LOF variants in LRRK2 increase or decrease the risk of PD.
Objective: To determine whether LRRK1 and LRRK2 LOF variants contribute to the risk of developing PD.
Design, setting, and participants: To determine the prevailing mechanism of LRRK2-mediated disease in human populations, next-generation sequencing data from a large case-control cohort (>23 000 individuals) was analyzed for LOF variants in LRRK1 and LRRK2. Data were generated at 5 different sites and 5 different data sets, including cases with clinically diagnosed PD and neurologically normal control individuals. Data were collected from 2012 through 2017.
Main outcomes and measures: Frequencies of LRRK1 and LRRK2 LOF variants present in the general population and compared between cases and controls.
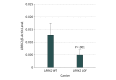
Results: Among 11 095 cases with PD and 12 615 controls, LRRK1 LOF variants were identified in 0.205% of cases and 0.139% of controls (odds ratio, 1.48; SE, 0.571; 95% CI, 0.45-4.44; P = .49) and LRRK2 LOF variants were found in 0.117% of cases and 0.087% of controls (odds ratio, 1.48; SE, 0.431; 95% CI, 0.63-3.50; P = .36). All association tests suggested lack of association between LRRK1 or LRRK2 variants and PD. Further analysis of lymphoblastoid cell lines from several heterozygous LOF variant carriers found that, as expected, LRRK2 protein levels are reduced by approximately half compared with wild-type alleles.
Conclusions and relevance: Together these findings indicate that haploinsufficiency of LRRK1 or LRRK2 is neither a cause of nor protective against PD. Furthermore, these results suggest that kinase inhibition or allele-specific targeting of mutant LRRK2 remain viable therapeutic strategies in PD.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS037167/NS/NINDS NIH HHS/United States
- MR/N026004/1/MRC_/Medical Research Council/United Kingdom
- G0701075/MRC_/Medical Research Council/United Kingdom
- G1100643/MRC_/Medical Research Council/United Kingdom
- WT089698/WT_/Wellcome Trust/United Kingdom
- MR/L010933/1/MRC_/Medical Research Council/United Kingdom
- 076113/WT_/Wellcome Trust/United Kingdom
- MR/L501554/1/MRC_/Medical Research Council/United Kingdom
- K-1501/PUK_/Parkinson's UK/United Kingdom
- WT089698/Z/09/Z/WT_/Wellcome Trust/United Kingdom
- ZIA NS003154/NS/NINDS NIH HHS/United States
- MR/L023784/2/MRC_/Medical Research Council/United Kingdom
- MR/G0901254/MRC_/Medical Research Council/United Kingdom
- R01 CA141668/CA/NCI NIH HHS/United States
- G-0907/PUK_/Parkinson's UK/United Kingdom
- Z99 AG999999/ImNIH/Intramural NIH HHS/United States
- H-1703/PUK_/Parkinson's UK/United Kingdom
- 083948/Z/07/Z/WT_/Wellcome Trust/United Kingdom
- MR/P005748/1/MRC_/Medical Research Council/United Kingdom
- Z01 AG000949/AG/NIA NIH HHS/United States
- 085475/WT_/Wellcome Trust/United Kingdom
- 090355/WT_/Wellcome Trust/United Kingdom
- G-1107/PUK_/Parkinson's UK/United Kingdom
- Z01 ES101986/ES/NIEHS NIH HHS/United States
- P50 NS071674/NS/NINDS NIH HHS/United States
- G0700943/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

