SGLT2 Inhibitors in Type 2 Diabetes Management: Key Evidence and Implications for Clinical Practice
- PMID: 30039249
- PMCID: PMC6167302
- DOI: 10.1007/s13300-018-0471-8
SGLT2 Inhibitors in Type 2 Diabetes Management: Key Evidence and Implications for Clinical Practice
Abstract
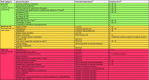
Management of type 2 diabetes mellitus (T2DM) is complex and challenging, particularly for clinicians working in primary care who are faced with many competing clinical priorities. The range of available T2DM treatments has diversified significantly in recent years, generating a busy and data-rich environment in which evidence is rapidly evolving. Sodium-glucose cotransporter-2 inhibitor (SGLT2i) agents are a relatively new class of oral glucose-lowering therapy that have been available in the UK for approximately 5 years. These agents reduce the reabsorption of glucose in the kidney and increase its excretion via the urine. Conflicting messages and opinions within the clinical community have led to misconceptions concerning the efficacy, safety and appropriate position of SGLT2i therapies within the T2DM treatment pathway. To help address some of these concerns and provide advice regarding the appropriate place of these medicines in clinical practice, the Improving Diabetes Steering Committee was formed. The Committee worked together to develop this review article, providing a summary of relevant data regarding the use of SGLT2i medicines and focusing on specific considerations for appropriate prescribing within the T2DM management pathway. In addition, a benefit/risk tool has been provided (see Fig. 3) that summarises many of the aspects discussed in this review. The tool aims to support clinicians in identifying the people most likely to benefit from SGLT2i treatments, as well as situations where caution may be required.
Funding: Napp Pharmaceuticals Limited.
Keywords: Clinical guidance; Glucose-lowering medicines; Oral glucose-lowering medicines; Prescribing tools; Risk/benefit; SGLT2 inhibitors; Therapy choice; Type 2 diabetes.
Figures


References
-
- Napp Pharmaceuticals Limited. Canagliflozin: summary of product characteristics. 2017. https://www.medicines.org.uk/emc/product/8855. Accessed April 2018.
-
- AstraZeneca UK Limited. Dapagliflozin: summary of product characteristics. 2017. https://www.medicines.org.uk/emc/product/7607. Accessed April 2018.
-
- Boehringer Ingelheim Limited. Empagliflozin: summary of product characteristics. 2018. https://www.medicines.org.uk/emc/product/5441. Accessed April 2018.
-
- National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. 2017. https://www.nice.org.uk/guidance/ng28. Accessed April 2018.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

