Achieving Controlled Biomolecule-Biomaterial Conjugation
- PMID: 30040387
- PMCID: PMC6107854
- DOI: 10.1021/acs.chemrev.8b00253
Achieving Controlled Biomolecule-Biomaterial Conjugation
Abstract
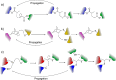
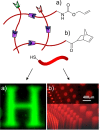
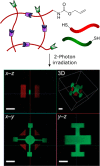
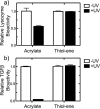
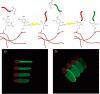
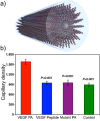
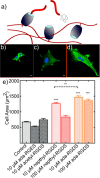
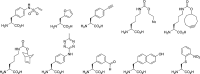
The conjugation of biomolecules can impart materials with the bioactivity necessary to modulate specific cell behaviors. While the biological roles of particular polypeptide, oligonucleotide, and glycan structures have been extensively reviewed, along with the influence of attachment on material structure and function, the key role played by the conjugation strategy in determining activity is often overlooked. In this review, we focus on the chemistry of biomolecule conjugation and provide a comprehensive overview of the key strategies for achieving controlled biomaterial functionalization. No universal method exists to provide optimal attachment, and here we will discuss both the relative advantages and disadvantages of each technique. In doing so, we highlight the importance of carefully considering the impact and suitability of a particular technique during biomaterial design.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



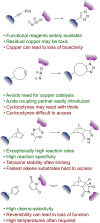
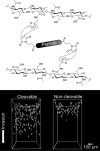
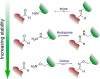

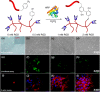
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

