Diffuse Amyloid-β Plaques, Neurofibrillary Tangles, and the Impact of APOE in Elderly Persons' Brains Lacking Neuritic Amyloid Plaques
- PMID: 30040735
- PMCID: PMC6537103
- DOI: 10.3233/JAD-180514
Diffuse Amyloid-β Plaques, Neurofibrillary Tangles, and the Impact of APOE in Elderly Persons' Brains Lacking Neuritic Amyloid Plaques
Abstract
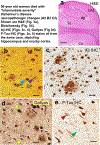
Data from a large autopsy series were analyzed to address questions pertinent to primary age-related tauopathy (PART) and Alzheimer's disease (AD): what factors are associated with increased severity of neurofibrillary degeneration in brains that lack neuritic amyloid plaques?; is there an association between Apolipoprotein E (APOE) alleles and PART pathologic severity independent of amyloid-β (Aβ) deposits?; and, how do the stains used to detect plaques and tangles impact the experimental results? Neuropathologic data were evaluated from elderly research volunteers whose brain autopsies were performed at University of Kentucky Alzheimer's Disease Center (UK-ADC; N = 145 subjects). All of the included subjects' brains lacked neuritic amyloid plaques according to the CERAD diagnostic criteria and the average final MMSE score before death was 26.8±4.6 stdev. The study incorporated evaluation of tissue with both silver histochemical stains and immunohistochemical stains to compare results; the immunohistochemical stains (Aβ and phospho-tau) were scanned and quantified using digital pathologic methods. Immunohistochemical stains provided important advantages over histochemical stains due to sensitivity and detectability via digital methods. When AD-type pathology was in its presumed earliest phases, neocortical parenchymal Aβ deposits were associated with increased medial temporal lobe neurofibrillary tangles. The observation supports the NIA-AA consensus recommendation for neuropathologic diagnoses, because even these "diffuse" Aβ deposits signal that AD pathobiologic mechanisms are occurring. Further, the data were most compatible with the hypothesis that the APOEɛ4 allele exerts its effect(s) via driving Aβ deposition, i.e., an "upstream" influence, rather than being associated directly with Aβ- independent PART pathology.
Keywords: Aging; Genie; MAPT; SNAP; ScanScope; amyloid-β; hippocampus; neuropathology; oldest-old.
Conflict of interest statement
Conflict of Interest/Disclosure Statement:
The authors have no conflict of interest to report
Figures







References
-
- Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, Abner EL, Markesbery WR (2007) Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol 66, 1136–1146. - PMC - PubMed
-
- Kovacs GG, Milenkovic I, Wohrer A, Hoftberger R, Gelpi E, Haberler C, Honigschnabl S, Reiner-Concin A, Heinzl H, Jungwirth S, Krampla W, Fischer P, Budka H (2013) Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta Neuropathol 126, 365–384. - PubMed
-
- Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Gearing M, Grinberg LT, Hof PR, Hyman BT, Jellinger K, Jicha GA, Kovacs GG, Knopman DS, Kofler J, Kukull WA, Mackenzie IR, Masliah E, McKee A, Montine TJ, Murray ME, Neltner JH, Santa-Maria I, Seeley WW, Serrano-Pozo A, Shelanski ML, Stein T, Takao M, Thal DR, Toledo JB, Troncoso JC, Vonsattel JP, White CL 3rd, Wisniewski T, Woltjer RL, Yamada M, Nelson PT(2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128, 755–766. - PMC - PubMed
-
- Bouras C, Hof PR, Morrison JH (1993) Neurofibrillary tangle densities in the hippocampal formation in a non-demented population define subgroups of patients with differential early pathologic changes. Neurosci Lett 153, 131–135. - PubMed
-
- Arriagada PV, Marzloff K, Hyman BT (1992) Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 42, 1681–1688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

