Real-time decision-making during emergency disease outbreaks
- PMID: 30040815
- PMCID: PMC6075790
- DOI: 10.1371/journal.pcbi.1006202
Real-time decision-making during emergency disease outbreaks
Abstract
In the event of a new infectious disease outbreak, mathematical and simulation models are commonly used to inform policy by evaluating which control strategies will minimize the impact of the epidemic. In the early stages of such outbreaks, substantial parameter uncertainty may limit the ability of models to provide accurate predictions, and policymakers do not have the luxury of waiting for data to alleviate this state of uncertainty. For policymakers, however, it is the selection of the optimal control intervention in the face of uncertainty, rather than accuracy of model predictions, that is the measure of success that counts. We simulate the process of real-time decision-making by fitting an epidemic model to observed, spatially-explicit, infection data at weekly intervals throughout two historical outbreaks of foot-and-mouth disease, UK in 2001 and Miyazaki, Japan in 2010, and compare forward simulations of the impact of switching to an alternative control intervention at the time point in question. These are compared to policy recommendations generated in hindsight using data from the entire outbreak, thereby comparing the best we could have done at the time with the best we could have done in retrospect. Our results show that the control policy that would have been chosen using all the data is also identified from an early stage in an outbreak using only the available data, despite high variability in projections of epidemic size. Critically, we find that it is an improved understanding of the locations of infected farms, rather than improved estimates of transmission parameters, that drives improved prediction of the relative performance of control interventions. However, the ability to estimate undetected infectious premises is a function of uncertainty in the transmission parameters. Here, we demonstrate the need for both real-time model fitting and generating projections to evaluate alternative control interventions throughout an outbreak. Our results highlight the use of using models at outbreak onset to inform policy and the importance of state-dependent interventions that adapt in response to additional information throughout an outbreak.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
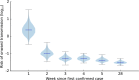
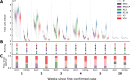
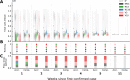
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

