The concerted amyloid-beta clearance of LRP1 and ABCB1/P-gp across the blood-brain barrier is linked by PICALM
- PMID: 30041013
- PMCID: PMC7748946
- DOI: 10.1016/j.bbi.2018.07.017
The concerted amyloid-beta clearance of LRP1 and ABCB1/P-gp across the blood-brain barrier is linked by PICALM
Abstract
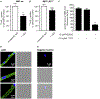
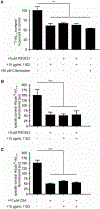
The accumulation of neurotoxic amyloid-beta (Aβ) in the brain is a characteristic hallmark of Alzheimer's disease (AD). The blood-brain barrier (BBB) provides a large surface area and has been shown to be an important mediator for removal of brain Aβ. Both, the ABC transporter P-glycoprotein (ABCB1/P-gp) and the receptor low-density lipoprotein receptor-related protein 1 (LRP1) have been implicated to play crucial roles in Aβ efflux from brain. Here, with immunoprecipitation experiments, co-immunostainings and dual inhibition of ABCB1/P-gp and LRP1, we show that both proteins are functionally linked, mediating a concerted transcytosis of Aβ through endothelial cells. Late-onset AD risk factor Phosphatidylinositol binding clathrin assembly protein (PICALM) is associated with both ABCB1/P-gp and LRP1 representing a functional link and guiding both proteins through the brain endothelium. Together, our results give more mechanistic insight on Aβ transport across the BBB and show that the functional interplay of different clearance proteins is needed for the rapid removal of Aβ from the brain.
Keywords: ABC transporter B1/P-glycoprotein (ABCB1/P-gp); Alzheimer’s disease (AD); Amyloid-beta (Aβ); Blood-brain barrier (BBB); Clearance; Endothelial cell; Endothelium; Low-density lipoprotein receptor-related protein 1 (LRP1); Phosphatidylinositol-binding clathrin assembly protein (PICALM); Transcytosis.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures


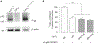





References
-
- Aleksis R, Oleskovs F, Jaudzems K, Pahnke J, Biverstal H, 2017. Structural studies of amyloid-beta peptides: unlocking the mechanism of aggregation and the associated toxicity. Biochimie 140, 176–192. - PubMed
-
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM, 2005. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J. Clin. Invest 115 (11), 3285–3290. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

