Clinical Pharmacokinetics in Kidney Disease: Application to Rational Design of Dosing Regimens
- PMID: 30042221
- PMCID: PMC6086693
- DOI: 10.2215/CJN.05150418
Clinical Pharmacokinetics in Kidney Disease: Application to Rational Design of Dosing Regimens
Abstract
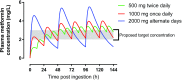
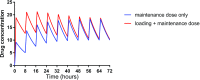
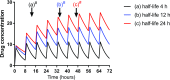
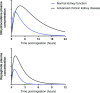
A change in pharmacokinetics can alter drug exposure and predispose the patient to either over- or underdosing, potentially resulting in adverse drug reactions or therapeutic failure. Kidney disease is characterized by multiple physiologic effects, which induce clinically significant changes in pharmacokinetics. These vary between individuals and may be quantitated in certain instances. An understanding of pharmacokinetic concepts is, therefore, important for a rational approach to the design of drug dosing regimens for the delivery of personalized medical care. Whether kidney disease is acute or chronic, drug clearance decreases and the volume of distribution may remain unchanged or increase. AKI is defined by dynamic changes in kidney function, which complicates attempts to accurately quantify drug clearance. In contrast, changes in drug clearance progress more slowly with CKD. In general, kidney replacement therapies increase drug clearance, but the extent to which this occurs depends on the modality used and its duration, the drug's properties, and the timing of drug administration. However, the changes in drug handling associated with kidney disease are not isolated to reduced kidney clearance and an appreciation of the scale of potential derangements is important. In most instances, the first dose administered in patients with kidney disease is the same as in patients with normal kidney function. However, in some cases, a higher (loading) initial dose is given to rapidly achieve therapeutic concentrations, followed by a lower maintenance dose, as is well described when prescribing anti-infectives to patients with sepsis and AKI. This review provides an overview of how pharmacokinetic principles can be applied to patients with kidney disease to personalize dosage regimens. Patients with kidney disease are a vulnerable population and the increasing prevalence of kidney disease means that these considerations are important for all prescribers.
Keywords: Acute Kidney Injury; Drug-Related Side Effects and Adverse Reactions; Pharmacokinetics; Prevalence; Renal Insufficiency, Chronic; Renal Replacement Therapy; Sepsis; Vulnerable Populations; acute kidney injury; chronic kidney disease; clearance; dialysis; kidney disease; personalized medicine; volume of distribution.
Copyright © 2018 by the American Society of Nephrology.
Figures
References
-
- Corsonello A, Pedone C, Lattanzio F, Onder G, Antonelli Incalzi R; Gruppo Italiano di Farmacovigilanza nell’Anziano (GIFA): Association between glomerular filtration rate and adverse drug reactions in elderly hospitalized patients: The role of the estimating equation. Drugs Aging 28: 379–390, 2011 - PubMed
-
- Zhang Y, Zhang L, Abraham S, Apparaju S, Wu TC, Strong JM, Xiao S, Atkinson AJ Jr, Thummel KE, Leeder JS, Lee C, Burckart GJ, Lesko LJ, Huang SM: Assessment of the impact of renal impairment on systemic exposure of new molecular entities: Evaluation of recent new drug applications. Clin Pharmacol Ther 85: 305–311, 2009 - PubMed
-
- Khanal A, Castelino RL, Peterson GM, Jose MD: Dose adjustment guidelines for medications in patients with renal impairment: How consistent are drug information sources? Intern Med J 44: 77–85, 2014 - PubMed
-
- Hassan Y, Al-Ramahi RJ, Aziz NA, Ghazali R: Impact of a renal drug dosing service on dose adjustment in hospitalized patients with chronic kidney disease. Ann Pharmacother 43: 1598–1605, 2009 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

